
ŹµŃéŹŅÖŠŠčŅŖÅäÖĘ500 mL 0.10 mol”¤L£1µÄNaOHČÜŅŗ£¬¾Ż“ĖŹµŃé»Ų“šĻĀĮŠĪŹĢā”£
(1)ÓĆĢģĘ½³ĘĮæNaOH¹ĢĢå________ g”£³ĘĮæNaOH¹ĢĢåŠč×¢ŅāŅŌĻĀĮ½øöĪŹĢā£ŗ¢ŁŅņĪŖNaOH¾ßÓŠøÆŹ“ŠŌ£¬ĖłŅŌ³ĘĮæŹ±£¬Šč×¢ŅāŃ”Ōń________Ź¢×°NaOH¹ĢĢ壻¢Ś³ĘĮæ±ŲŠėŃøĖŁ£¬ŌŅņŹĒ________________________________________________________________________
________________________________________________________________________ӣ
(2)ŹµŃéĖłŠčŅŖµÄŅĒĘ÷ÓŠČŻĮæĘæ(¹ęøńŹĒ______)£¬»¹ÓŠ_________________________”£
(3)ĻĀĮŠ²Ł×÷¶ŌĖłÅäÖʵÄČÜŅŗµÄÅضČƻӊӰĻģµÄŹĒ(””””)
A£®³ĘĮæŹ±ŅŃ¹Ū²ģµ½NaOHĪüĖ®
B£®½«ÉÕ±ÖŠČܽāŗóµÄČÜŅŗŃøĖŁ×¢ČėČŻĮæĘæ£¬Č»ŗóŌŁµĪ¼ÓÕōĮóĖ®ÖĮæĢ¶ČĻß
C£®Ņ”ŌȶØČŻŹ±£¬ÓĆ½ŗĶ·µĪ¹ÜĻņČŻĮæĘæÖŠµĪ¼ÓÕōĮóĖ®ÖĮæĢ¶ČĻß
D£®ÅäÖĘČÜŅŗĒ°ÓĆÕōĮóĖ®ČóĻ“ČŻĮæĘæ
 ÖĒ»ŪŠ”ø“Ļ°ĻµĮŠ“š°ø
ÖĒ»ŪŠ”ø“Ļ°ĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠ»Æѧ·“Ó¦µÄĄė×Ó·½³ĢŹ½ÕżČ·µÄŹĒ(””””)
A£®½šŹōFeÓėĻ”H2SO4·“Ó¦£ŗFe + 2H+ == Fe3+ + H2”ü
B£®Al(OH)3ÖĪĮĘĪøĖį¹ż¶ą£ŗ H£«£«OH”Ŗ===H2O
C£®NaHCO3ČÜŅŗÓėNaOHČÜŅŗ»ģŗĻ£ŗHCO3”Ŗ+OH”Ŗ ==H2O + CO2”ü
D£®AlÓėNaOHČÜŅŗ·“Ó¦£ŗ2Al£«2OH££«2H2O===2AlO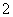 £«3H2”ü
£«3H2”ü
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
W”¢X”¢Y”¢ZŹĒŌ×ÓŠņŹżŅĄ“ĪŌö“óµÄĶ¬Ņ»¶ĢÖÜĘŚŌŖĖŲ£¬W”¢XŹĒ½šŹōŌŖĖŲ£¬Y”¢ZŹĒ·Ē½šŹōŌŖĖŲ”£
(1)W”¢Xø÷×ŌµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļæÉŅŌ·“Ӧɜ³ÉŃĪ£¬øĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗ________________________________________________________________________”£
(2)WÓėYæÉŠĪ³É»ÆŗĻĪļW2Y£¬øĆ»ÆŗĻĪļµÄ»ÆѧŹ½ĪŖ£ŗ__________________________”£
(3)±Č½ĻY”¢ZĘųĢ¬Ēā»ÆĪļµÄĪČ¶ØŠŌ£ŗ________>________(ÓĆ»ÆѧŹ½±ķŹ¾)”£
(4)W”¢X”¢Y”¢ZĖÄÖÖŌŖĖŲ¼ņµ„Ąė×ӵĥė×Ó°ė¾¶Óɓ󵽊”µÄĖ³ŠņŹĒ£ŗ_______(ÓĆ»ÆѧŹ½±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijŗÓµĄĮ½ÅŌÓŠ¼×”¢ŅŅĮ½³§”£ĖüĆĒÅŷŵŤŅµ·ĻĖ®ÖŠ£¬¹²ŗ¬K£«”¢Ag£«”¢Fe3£«”¢Cl£”¢OH£”¢NO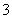 ĮłÖÖĄė×Ó”£
ĮłÖÖĄė×Ó”£
(1)¼×³§µÄ·ĻĖ®Ć÷ĻŌ³Ź¼īŠŌ£¬¹Ź¼×³§·ĻĖ®ÖŠĖłŗ¬ÓŠµÄČżÖÖĄė×ÓŹĒ________”¢________”¢________”£
(2)ŅŅ³§µÄ·ĻĖ®ÖŠŗ¬ÓŠĮķĶāČżÖÖĄė×Ó”£Čē¹ū¼ÓŅ»¶ØĮæµÄ________(Ń”Ģī£ŗ”°»īŠŌĢæ”±”¢”°ĮņĖįŃĒĢś”±”¢”°Ģś·Ū”±)£¬æÉŅŌ»ŲŹÕĘäÖŠµÄ½šŹō________(ĢīŠ“½šŹōŌŖĖŲ·ūŗÅ)”£
(3)ĮķŅ»ÖÖÉčĻėŹĒ½«¼×³§ŗĶŅŅ³§µÄ·ĻĖ®°“ŹŹµ±µÄ±ČĄż»ģŗĻ£¬æÉŅŌŹ¹·ĻĖ®ÖŠµÄ__________(ĢīŠ“Ąė×Ó·ūŗÅ)×Ŗ»ÆĪŖ³Įµķ”£¾¹żĀĖŗóµÄ·ĻĖ®Ö÷ŅŖŗ¬____________(ĢīŠ“»ÆѧŹ½)£¬æÉÓĆĄ“½½¹ąÅ©Ģļ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
½«5 mol”¤L£1µÄMg(NO3)2ČÜŅŗa mLĻ”ŹĶÖĮb mL£¬Ļ”ŹĶŗóČÜŅŗÖŠNO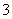 µÄĪļÖŹµÄĮæÅضČĪŖ(””””)
µÄĪļÖŹµÄĮæÅضČĪŖ(””””)
A.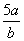 mol”¤L£1 B.
mol”¤L£1 B.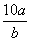 mol”¤L£1
mol”¤L£1
C.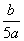 mol”¤L£1 D.
mol”¤L£1 D.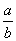 mol”¤L£1
mol”¤L£1
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚ“ÖŃĪĢį“æµÄŹµŃéÖŠ£¬Õō·¢Ź±ÕżČ·µÄ²Ł×÷ŹĒ£ŗ
A£®°Ń»ė×ĒµÄŅŗĢåµ¹ČėÕō·¢ĆóÄŚ¼ÓČČ B£®æŖŹ¼Īö³ö¾§ĢåŗóÓĆ²£Į§°ō½Į°č
C£®“żĖ®·ÖĶźČ«ÕōøÉŗóĶ£Ö¹¼ÓČČ D£®Õō·¢ĆóÖŠ³öĻÖ“óĮæ¹ĢĢåŹ±¼“Ķ£Ö¹¼ÓČČ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŌŚŹµŃéŹŅÖŠ£¬“ÓµāĖ®ÖŠĢįČ”µāµÄ²Ł×÷£¬ÕżČ·µÄŹĒ£Ø £©
A.·ÖŅŗ”¢ÕōĮó B.Čܽā”¢¹żĀĖ”¢Õō·¢ C.ŻĶČ””¢·ÖŅŗ D.ŻĶČ””¢·ÖŅŗ”¢ÕōĮó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀĮŠø÷×éĪļÖŹ³ä·Ö·“Ó¦ŗó£¬Ö»ÄܵƵ½Ņ»ÖÖĘųĢåµÄŹĒ
A£®Ä¾ĢæŗĶÅØĮņĖį¹²ČČ
B£®ÄĘÓėĖ®·“Ó¦
C£®×ćĮæµÄĶøśŅ»¶ØĮæµÄÅØĻõĖį·“Ó¦
D£®ĘūÓĶŌŚĘū³µ·¢¶Æ»śČ¼ÉÕŗóÅųöµÄĘųĢå
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓƶčŠŌµē¼«µē½ā±„ŗĶNa2CO3ČÜŅŗ£¬Čō±£³ÖĪĀ¶Č²»±ä£¬ŌņŅ»¶ĪŹ±¼äŗó
A£®ČÜŅŗµÄpH±ä“ó B£®c(Na£«)Óėc(CO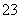 )µÄ±ČÖµ±ä“ó
)µÄ±ČÖµ±ä“ó
C£®ČÜŅŗÅØ¶Č±ä“ó£¬ÓŠ¾§ĢåĪö³ö D£®ČÜŅŗÅØ¶Č²»±ä£¬ÓŠ¾§ĢåĪö³ö
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com