
Č”50.0 mL Na2CO3ŗĶNa2SO4µÄ»ģŗĻČÜŅŗ£¬¼ÓČė¹żĮæBaCl2ČÜŅŗŗóµĆµ½14.51 g°×É«³Įµķ£¬ÓĆ¹żĮæĻ”ĻõĖį“¦Ąķŗó³ĮµķĮæ¼õÉŁµ½4.66 g £¬²¢ÓŠĘųĢå·Å³ö”£
(1)ÓĆĻ”ĻõĖį“¦ĄķŗóµÄ²»ČÜĪļĪŖ______________(Ģī»ÆѧŹ½)£¬Š“³öÕūøö¹ż³ĢÖŠĖł·¢Éś·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ__________________£¬__________________£¬__________________”£
(2)Ō»ģŗĻČÜŅŗÖŠNa2CO3ŗĶNa2SO4µÄĪļÖŹµÄĮæÅØ¶Č·Ö±šŹĒ________”¢________”£
(3)²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żĪŖ________”£
½āĪö””(2)×īŗóŹ£Óą³ĮµķĪŖBaSO4
n(Na2SO4)£½n(BaSO4)£½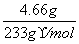 £½0.02mol
£½0.02mol
c(Na2SO4)£½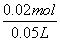 £½0.4mol”¤L£1
£½0.4mol”¤L£1
n(Na2CO3)£½n(BaCO3)£½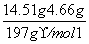 £½0.05mol
£½0.05mol
c(Na2CO3)£½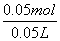 £½1mol”¤L£1
£½1mol”¤L£1
(3)V(CO2)£½0.05mol”Į22.4L”¤mol£1£½1.12L”£
“š°ø””(1)BaSO4
CO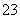 £«Ba2£«===BaCO3”ż
£«Ba2£«===BaCO3”ż
SO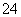 £«Ba2£«===BaSO4”ż
£«Ba2£«===BaSO4”ż
BaCO3£«2H£«===Ba2£«£«H2O£«CO2”ü
(2)0.4 mol”¤L£1
1£®0 mol”¤L£1
(3)1.12 L



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Äܹ»²ā¶ØÓŠ»śĪļ·Ö×ÓÖŠ»Æѧ¼üŗĶ¹ŁÄÜĶÅµÄŹĒ£Ø £©
A”¢ÖŹĘ× B”¢ŗģĶā¹āĘ× C”¢×ĻĶā¹āĘ× D”¢ŗĖ“Ź²ÕńĘ×
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĄūÓĆÉś»īÖŠ³£¼ūµÄ²ÄĮĻæÉŅŌ½ųŠŠŗܶąæĘѧŹµŃ飬ÉõÖĮÖĘ×÷³öŅ»Š©ÓŠŹµ¼ŹÓ¦ÓĆ¼ŪÖµµÄ×°ÖĆĄ“£¬
Čē·ĻĀĮ¹ŽŗĶĢ¼°ō£¬Ź³ŃĪĖ®µČ²ÄĮĻÖĘ×÷æÉÓĆÓŚĒż¶ÆĶę¾ßµÄµē³Ų”£ÉĻŹöµē³Ų¹¤×÷Ź±£¬
ÓŠ¹ŲĖµ·ØÕżČ·µÄŹĒ £Ø £©
A£®ĀĮ¹Ž½«Öš½„±»øÆŹ“
B£®Ģ¼°ōÉĻ·¢ÉśµÄ·“Ó¦ĪŖ£ŗO2£«4e£===2O2£
C£®Ģ¼°ōÓ¦ÓėĶę¾ßµē»śµÄøŗ¼«ĻąĮ¬
D£®øƵē³Ų¹¤×÷Ņ»¶ĪŹ±¼äŗóĢ¼°ōµÄÖŹĮæ»į¼õĒį
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŖĮĖ¼ģŃéNa2SO3ÖŠŹĒ·ń»ģÓŠNa2SO4£¬Ó¦Ń”ÓƵďŌ¼ĮŹĒ(””””)
A£®BaCl2ČÜŅŗ B£®BaCl2ČÜŅŗŗĶĻ”ŃĪĖį
C£®BaCl2ČÜŅŗŗĶĻ”ĻõĖį D£®BaCl2ČÜŅŗŗĶĻ”ĮņĖį
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijĶ¬Ń§ĪŖ¼ģŃéČÜŅŗÖŠŹĒ·ńŗ¬ÓŠ³£¼ūµÄĖÄÖÖĪŽ»śĄė×Ó£¬½ųŠŠĮĖĻĀĶ¼ĖłŹ¾µÄŹµŃé²Ł×÷”£ĘäÖŠ¼ģŃé¹ż³ĢÖŠ²śÉśµÄĘųĢåÄÜŹ¹ŗģÉ«ŹÆČļŹŌÖ½±äĄ¶”£ÓÉøĆŹµŃéÄܵƵ½µÄÕżČ·½įĀŪŹĒ(””””)
A£®ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠSO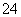
B£®ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠNH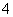 Ąė×Ó
Ąė×Ó
C£®ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠCl£Ąė×Ó
D£®ŌČÜŅŗÖŠŅ»¶Øŗ¬ÓŠFe3£«Ąė×Ó
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
°Ń0.05 mol NaOH¹ĢĢå·Ö±š¼ÓČėĻĀĮŠ100 mLČÜŅŗÖŠ£¬ČÜŅŗµÄµ¼µēŠŌ»ł±¾²»±äµÄŹĒ
A£®×ŌĄ“Ė® B£®0.5 mol/LŃĪĖį
C£®0.5 mol/L“×Ėį D£®0.5 mol/L mol·L”„1°±Ė®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
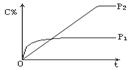
ŌŚĪĀ¶ČĻąĶ¬£¬Ń¹Ēæ·Ö±šĪŖp1”¢p2Ģõ¼žĻĀ£¬A(g)£«2B(g)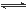 nC(g)µÄ·“Ó¦ĢåĻµÖŠ,CµÄĢå»ż·ÖŹż(C£„)Ėꏱ¼ä(t)±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£®
nC(g)µÄ·“Ó¦ĢåĻµÖŠ,CµÄĢå»ż·ÖŹż(C£„)Ėꏱ¼ä(t)±ä»ÆµÄĒśĻßČēĶ¼ĖłŹ¾£®
ĻĀĮŠ½įĀŪÕżČ·µÄŹĒ
A£®p1 > p2 n < 3 B£®p1 < p2 n > 3
C£®p1 < p2 n £½ 3 D£®p1 > p2 n > 3
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ÓĆNA±ķŹ¾°¢·ü¼ÓµĀĀŽ³£Źż£¬ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ( )
A£®±ź×¼×“æöĻĀ£¬22.4LCCl4ŗ¬ÓŠµÄ·Ö×ÓŹżĪŖNA
B£®³£ĪĀ³£Ń¹ĻĀ£¬17gNH3 Ėłŗ¬µÄŌ×ÓŹżÄæĪŖ4NA
C£®1 mol Na2O2ÓėH2OĶźČ«·“Ó¦£¬×ŖŅĘ2NAøöµē×Ó
D£®0.1mol/LNa2CO3ČÜŅŗÖŠŗ¬ÓŠµÄNa+ŹżÄæĪŖ0.2NA
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
Ģ¼ŗĶµŖµÄŠķ¶ą»ÆŗĻĪļŌŚ¹¤Å©ŅµÉś²śŗĶÉś»īÖŠÓŠÖŲŅŖµÄ×÷ÓĆ”£
£Ø1£©ŅŌCO2ÓėNH3ĪŖŌĮĻæÉŗĻ³É»Æ·ŹÄņĖŲ[»ÆѧŹ½ĪŖCO(NH2)2]”£ŅŃÖŖ£ŗ
¢Ł2NH3(g)£«CO2(g) === NH2CO2NH4(s) ∆H£½-159.5 kJ”¤mol£1
¢ŚNH2CO2NH4(s) === CO(NH2)2(s)£«H2O(g) ∆H£½+116.5 kJ”¤mol£1
¢ŪH2O(l) === H2O(g) ∆H£½+44.0 kJ”¤mol£1
Ōņ·“Ó¦2NH3(g)£«CO2(g) === CO(NH2)2(s)£«H2O(l)µÄ∆H£½ kJ”¤mol£1
£Ø2£©ÓĆ»īŠŌĢ滹Ō·ØæÉŅŌ“¦ĄķµŖŃõ»ÆĪļ”£Ä³ŃŠ¾æŠ”×éĻņijĆܱÕČŻĘ÷¼ÓČėŅ»¶ØĮæµÄ»īŠŌĢæŗĶNO£¬·¢Éś·“Ó¦C(s)£«2NO(g) N2(g)£«CO2 (g) ∆H£½Q kJ”¤mol£1”£
N2(g)£«CO2 (g) ∆H£½Q kJ”¤mol£1”£
ŌŚT1”ꏱ£¬·“Ó¦½ųŠŠµ½Ķ¬Ź±¼ä²āµĆø÷ĪļÖŹµÄÅضČČēĻĀ£»30minŗó£¬Ö»øıäijŅ»Ģõ¼ž£¬·“Ó¦ÖŲŠĀ“ļµ½Ę½ŗā£¬ø÷ĪļÖŹÅضČČēĶ¼Ėł”£
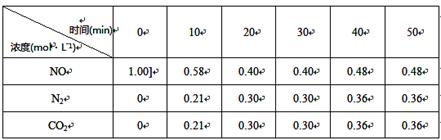
¢Ł0~10minÄŚ£¬NOµÄĘ½¾ł·“Ó¦ĖŁĀŹv(NO)£½ £¬
T1”ꏱ£¬øĆ·“Ó¦µÄĘ½ŗā³£ŹżK£½ £»
¢Ś30minŗó£¬Ö»øıäijŅ»Ģõ¼ž£¬·“Ó¦ÖŲŠĀ“ļµ½Ę½ŗā£¬ø÷ĪļÖŹÅضČČēĶ¼ĖłŹ¾”£øł¾ŻÉĻ±ķÖŠµÄŹż¾ŻÅŠ¶ĻøıäµÄĢõ¼žæÉÄÜŹĒ £ØĢī×ÖÄø±ąŗÅ£©”£
a£®¼ÓČėŅ»¶ØĮæµÄ»īŠŌĢæ b£®ĶØČėŅ»¶ØĮæµÄNO
c£®ŹŹµ±ĖõŠ”ČŻĘ÷µÄĢå»ż d£®¼ÓČėŗĻŹŹµÄ“߻ƼĮ e£®ÉżøßĪĀ¶Č
¢Ū30minŗó£¬Čē¹ū½«ĪĀ¶ČÉżøßÖĮT2”ę£¬“ļµ½Ę½ŗāŹ±£¬ČŻĘ÷ÖŠNO”¢N2”¢CO2µÄÅضČÖ®±ČĪŖ5:3:3£¬Ōņ Q 0£ØĢī”°£¾”±»ņ”°£¼”±£©”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com