
| V/s | 50 | 100 | |
| c��N2O3��/mol?L-1 | 5.00 | 3.52 | 2.48 |

 �����㣻
�����㣻 ��180.5kJ?mol-1×2+92.4kJ?mol-1×2-483.6×3��=226.3 kJ?mol-1���ʴ�Ϊ��226.3 kJ��
��180.5kJ?mol-1×2+92.4kJ?mol-1×2-483.6×3��=226.3 kJ?mol-1���ʴ�Ϊ��226.3 kJ�� 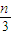 ����n=3mol��Ӧ�ﵽƽ��ʱ��H2��ת����Ϊ60%������ʼ����Ũ��Ϊ1mol/l���仯������Ũ��Ϊ1.8mol/l���仯�ĵ���Ũ��Ϊ0.6mol/l��ƽ��ʱ������������������Ũ�ȷֱ���0.4mol/l��1.2mol/l��1.2mol/l����k=
����n=3mol��Ӧ�ﵽƽ��ʱ��H2��ת����Ϊ60%������ʼ����Ũ��Ϊ1mol/l���仯������Ũ��Ϊ1.8mol/l���仯�ĵ���Ũ��Ϊ0.6mol/l��ƽ��ʱ������������������Ũ�ȷֱ���0.4mol/l��1.2mol/l��1.2mol/l����k=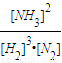 =
=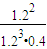 =2.08 ��mol?L-1��-2���ʴ�Ϊ��
=2.08 ��mol?L-1��-2���ʴ�Ϊ��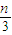 �� 2.08��mol?L-1��-2��
�� 2.08��mol?L-1��-2�� =
=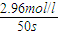 =0.0592mol?L-1?s-1���ʴ�Ϊ��0.0592mol?L-1?s-1��
=0.0592mol?L-1?s-1���ʴ�Ϊ��0.0592mol?L-1?s-1�� 

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺| t/s | 0 | 500 | 1000 |
| c��N2O5��/mol?L-1 | 5.00 | 3.52 | 2.48 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺| t/s | 0 | 500 | 1000 |
| c��N2O5��/mol?L-1 | 5.00 | 3.52 | 2.48 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
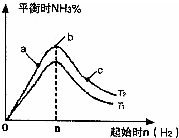 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| n |
| 3 |
| n |
| 3 |
| V/s | 0 | 50 | 100 |
| c��N2O3��/mol?L-1 | 5.00 | 3.52 | 2.48 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
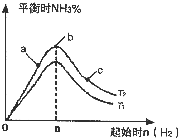 ����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺
����N2��H2����ʵ��NH3�Ĺ�ҵ�ϳɣ������ֿ��Խ�һ���Ʊ����ᣬ�ڹ�ҵ��һ��ɽ���������������ش��������⣺�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com