
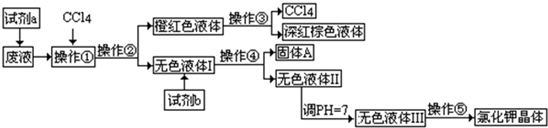
ij�����ķ�Һ�к��д�����K+��Cl����Br��������������Ca2+��Mg2+��SO42����ij�о���ѧϰС���������ַ�Һ����ȡ�ϴ������Ȼ��ؾ��弰Һ�壨Br2��������������������̣�
�ɹ��Լ�a��b��ѡ���Լ�������Na2CO3��Һ������K2CO3��Һ��KO H��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��KMnO4(H+)��Һ��ϡHNO3��
H��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��KMnO4(H+)��Һ��ϡHNO3��
������������̣��ش�������⣺
���Լ�aӦ��ѡ��_______________��
�Ʋ����١��ڡ��ۡ��ܡ��ݵ�������_________(����ĸ)��
| A����ȡ�����ˡ���Һ�����ˡ������ᾧ | B����ȡ����Һ�������ˡ������ᾧ |
| C����Һ����ȡ�����ˡ����ˡ������ᾧ | D����ȡ����Һ����Һ�����ˡ������ᾧ |
 �������� ������������ ��
�������� ������������ �� ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д� �����Ծ�ϵ�д�
�����Ծ�ϵ�д� �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ����һ��2012������������¿���ѧ����(�˽̰�) ���ͣ�058
| |||||||||||||||||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�����ķ�Һ�к��д�����K+��Cl����Br��������������Ca2+��Mg2+��SO42����ij�о���ѧϰС������ȡ���ַ�Һ����ȡ�ϴ������Ȼ��ؾ��弰Һ�壨Br2��������������������̣�
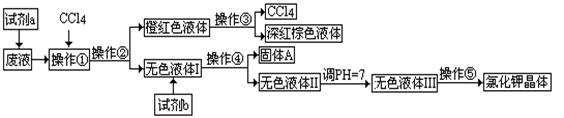
�ɹ��Լ�a��b��ѡ���Լ�������Na2CO3��Һ������K2CO3��Һ��KOH��Һ��BaCl2��Һ��Ba(NO3)2��Һ��H2O2��KMnO4(H+)��Һ��ϡHNO3��
������������̣��ش�������⣺
��1���Լ�aӦ��ѡ��_______________��
��2�������١��ڡ��ۡ��ܡ��ݵ�������_________(����ĸ)��
A����ȡ�����ˡ���Һ�����ˡ������ᾧ B����ȡ����Һ�������ˡ������ᾧ
C����Һ����ȡ�����ˡ����ˡ������ᾧ D����ȡ����Һ����Һ�����ˡ������ᾧ
��3����ȥ��ɫҺ��I�е�Ca2+��Mg2+��SO42-���ӣ�ѡ��C���������Լ������μ�˳�������� ���ѧʽ����
��4������pH�������� ������������ ��
��5�����������õ��Ĵ������������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com