
| ʵ����� | �� | �� | �� |
| �μ���ҺA�����/mL | 10.0 | 20.0 | 30.0 |
| ������������/mL����״���� | 89.6 | 179.2 | 224 |
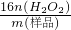 ��100%]��������̼���Ʋ�Ʒ�����ӣ�13%������Ϊ�ŵ�Ʒ���ֽ�0.2gij���������Ĺ�̼������Ʒ���������ʲ���������������ԭ��Ӧ������ˮ�����Һ������15.0mL 1mol/L���ᣬ�ټ�������KI��ҡ�Ⱥ����ڰ�������ַ�Ӧ��������������Һ����0.1mol/L Na2S2O3��Һ�ζ�����ɫǡ����ʧʱ��������30.00mL���Լ����жϸ���Ʒ�Ƿ�Ϊ�ŵ�Ʒ������֪��2Na2S2O3+I2��Na2S4O6+2NaI��
��100%]��������̼���Ʋ�Ʒ�����ӣ�13%������Ϊ�ŵ�Ʒ���ֽ�0.2gij���������Ĺ�̼������Ʒ���������ʲ���������������ԭ��Ӧ������ˮ�����Һ������15.0mL 1mol/L���ᣬ�ټ�������KI��ҡ�Ⱥ����ڰ�������ַ�Ӧ��������������Һ����0.1mol/L Na2S2O3��Һ�ζ�����ɫǡ����ʧʱ��������30.00mL���Լ����жϸ���Ʒ�Ƿ�Ϊ�ŵ�Ʒ������֪��2Na2S2O3+I2��Na2S4O6+2NaI�� 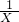 =
=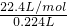
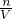 =
=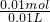 =1 mol/L��
=1 mol/L��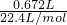 =0.03mol��n��H2O2��=0.06mol����ʵ��������ݿ�֪��10mLA��Һ�У�n��Na2CO3��=n��CO2��=0.004 mol������100mLA�к���0.04molNa2CO3��n��Na2CO3����n��H2O2��=0.04��0.06=2��3
=0.03mol��n��H2O2��=0.06mol����ʵ��������ݿ�֪��10mLA��Һ�У�n��Na2CO3��=n��CO2��=0.004 mol������100mLA�к���0.04molNa2CO3��n��Na2CO3����n��H2O2��=0.04��0.06=2��3 =
= =1.5��10-3mol��
=1.5��10-3mol��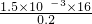 =12%��13%�����Ը���Ʒ�����ŵ�Ʒ��
=12%��13%�����Ը���Ʒ�����ŵ�Ʒ�� ��
��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��㶫ʡ������ѧ�����п������ۻ�ѧ�Ծ��������棩 ���ͣ������
�м���ʵ��С���ͬѧ��������ͼװ�ý��С�һ�����á���ʵ��̽��(a��ʢ�ŵ�Һ��������ٵ��£�b��ʢ�ŵ�ҩƷ����������c��d��ʢװҺ�壬���ܾ�����Һ������)��ÿ��ͬѧ������a��b��c��d�зֱ�ʢ�Ų�ͬ���ʣ�����ȡij�����岢���������ʡ�

����ش����¸���ͬѧ�ڽ���ʵ����Ʒ�������������⣺
��.��1����a��Ũ���b���������(�������Աȶ�������ǿ�ܶ�)��c����ɫ�ʻ��ꡣ��ʵ�������c�е�����_____________________________________________________________��
dװ����ʢ��ҩƷ��������______________________________����д��d�з�Ӧ�����ӷ���ʽ�� ___________________________________________________________________��
��2����a��ϡ���b�����Ƿۣ�c������̼������Һ��d������Na2SiO3��Һ����ʵ���
���У�c�г��ֵ�������____________________��d��������____________����˵��
________________________________________________________________________��
��.����Ϊ����ͬѧ����ȡ����֮ǰ��Ӧ���еIJ�����________________���㻹�������ô�װ�õ�a��b��������ȡ��������(ֻдһ��)_________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com