
 ��4.18��10 -4 mol?L-1 ���� ��5��11:1
��4.18��10 -4 mol?L-1 ���� ��5��11:1 CH3COO-+H+
CH3COO-+H+ 

 ��ѧ����ͬ����ϰϵ�д�
��ѧ����ͬ����ϰϵ�д� ��ǰ�κ�ͬ����ϰϵ�д�
��ǰ�κ�ͬ����ϰϵ�д� ����С��ҵϵ�д�
����С��ҵϵ�д� �Ƹ�С״Ԫ����������ϰ��ϵ�д�
�Ƹ�С״Ԫ����������ϰ��ϵ�д� �ɹ�ѵ���ƻ�ϵ�д�
�ɹ�ѵ���ƻ�ϵ�д� ����ѵ����ֱͨ�п�����ϵ�д�
����ѵ����ֱͨ�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 H����F����25���£���20mL0.2mol?L-1��������еμ�0.2mol?L-1��NaOH��Һʱ����Һ��pH�仯��ͼ��ʾ��
H����F����25���£���20mL0.2mol?L-1��������еμ�0.2mol?L-1��NaOH��Һʱ����Һ��pH�仯��ͼ��ʾ��
 ���͵���ƽ�ⳣ����K��һ�������������������������ϡ��Һ�еĵ���������
���͵���ƽ�ⳣ����K��һ�������������������������ϡ��Һ�еĵ��������� ��
�� (HF)]�������_______����ʹ�����ĵ���ƽ�ⳣ��[Ka(HF)] �������_______��
(HF)]�������_______����ʹ�����ĵ���ƽ�ⳣ��[Ka(HF)] �������_______�� ԼΪ___ _%��
ԼΪ___ _%�� H��(aq)��F��(aq) ��H����b kJ?mol -1
H��(aq)��F��(aq) ��H����b kJ?mol -1 = Ka(HF)
= Ka(HF)�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
A��H2S 2H�� + S2�� 2H�� + S2�� | B��HClO = H�� +ClO�� |
C��CH3COONH4 NH4+ +CH3COO�� NH4+ +CH3COO�� | D��HCO3��+H2O  H3O++CO32- H3O++CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaF | B��NH4Cl | C��HClO | D��HNO3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| | þ������ | ���� | ��ʼ�ķ�Ӧ���� | ����H2���� |
| �� | 0.24g | HCl�� 0.2 mol/L 100mL | ��1 | n1 |
| �� | 0.24g | CH3COOH��0.2 mol/L 100mL | ��2 | n2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
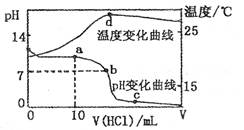
A��a����Һ������Ũ�ȴ�С�Ĺ�ϵ��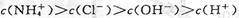 |
B��b����Һ������Ũ�ȴ�С�Ĺ�ϵ��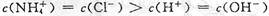 |
C��c����Һ������Ũ�ȴ�С�Ĺ�ϵ��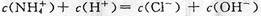 |
| D��d��ʱ��Һ�¶ȴﵽ��ߣ�֮���¶������½���ԭ����NH3? H2O�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�������������ʯ��Ӧ����������̼���� |
| B��pH = 3�Ĵ�����Һϡ��100����pH<5 |
| C�������£���������Һ��pH>7 |
| D�������£�0.1 mol��L��1������ҺpH="2.5" |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������� | B����Է������� |
| C������̶� | D���ܽ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com