
ij�л���X��C12H13O6Br�������к��ж��ֹ����ţ���ṹ��ʽΪ ������I��IIΪδ֪���ֵĽṹ��
������I��IIΪδ֪���ֵĽṹ��
Ϊ�Ʋ�X�ķ��ӽṹ��������ͼ��ת����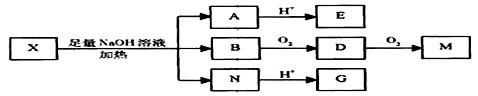
��֪��E��ˮ��Һ�е���FeCl3��Һ������ɫ��Ӧ��M��C2H2O4����ʹ��īˮ��ɫ��G��M������NaHCO3��Һ��Ӧ����ش�
��1��M�Ľṹ��ʽΪ ��G�������������ŵ������� ��
��2��E���Է����ķ�Ӧ�У�ѡ����ţ� ��
�ټӳɷ�Ӧ ����ȥ��Ӧ ��������Ӧ ��ȡ����Ӧ
��3��G��һ�������·�����Ӧ���ɷ������ΪC4H4O4���л�����л����ʹ������Ȼ�̼��Һ��ɫ����д��G�����˷�Ӧ�Ļ�ѧ����ʽ
��
��4����֪��X���ӽṹ�У�I�ṹ��������FeCl3��Һ������ɫ��Ӧ�Ĺ����ţ���E�����б����ϵ�һ�ȴ���ֻ��һ�֣���X�Ľṹ��ʽ�� ��
��5��F��G��Ϊͬ���칹�壬F�ķ�����ֻ�����Ȼ����ǻ���ȩ�����ֹ����ţ���ͬһ��̼ԭ���ϲ���ͬʱ���������ǻ�����F�ķ��ӽṹ����Ϊ ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


 Z+H2O��д���÷�Ӧ�Ļ�ѧ����ʽ
Z+H2O��д���÷�Ӧ�Ļ�ѧ����ʽ



�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������������غ㶨�ɣ����жϳ���һ����Ӧ����Ϊ
�������������غ㶨�ɣ����жϳ���һ����Ӧ����Ϊ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij�л���X�ļ���ʽΪ��
ij�л���X�ļ���ʽΪ��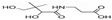 �������л���X��˵����ȷ���ǣ�������
�������л���X��˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���٢ڢ� | B���٢ۢ� | C���٢ڢ� | D���٢ڢۢܢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com