
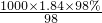 mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL������xmL��18.4mol/L=500mL��0.2mol/L����ã�x��5.4������Ӧ��ȡ��Ũ���������5.4mL��
mol/L=18.4mol/L������ϡ�Ͷ��ɣ�ϡ��ǰ�����ʵ����ʵ������䣬������Ũ������������Ũ��������ΪxmL������xmL��18.4mol/L=500mL��0.2mol/L����ã�x��5.4������Ӧ��ȡ��Ũ���������5.4mL�� ���ٸ�����Һϡ��ǰ�����ʵ��������������Ũ����������
���ٸ�����Һϡ��ǰ�����ʵ��������������Ũ����������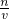 �жϣ�
�жϣ�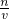 �������������ʵ����ʵ��������Һ�������Ӱ���ж�
�������������ʵ����ʵ��������Һ�������Ӱ���ж� 

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ����Ҫ����1mol/L��ϡ����250mL���ش��������⣺
�� ��Ҫ98%�ܶ�Ϊ1.84g/cm3��Ũ���� mL��
�� ����ʱ������ʹ�õ������� 20 mL��Ͳ�� 250 mL����ƿ���������⣬����Ҫ�������� ������ ��
�� ����ʱ����ʵ�������õ��������������÷ֱ��� �� ��
�� ���ƹ����г��������������������ҺŨ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡���Ӱ�족��û��ϴ���ձ��Ͳ�����������������������ƿû�и���������������
��2��������ͼ��ʾװ�ã��г�����ʡ�ԣ��ݲ�����β�������գ�����ʵ�飬��Һ��A��μ��뵽����B�У��ش��������⣺
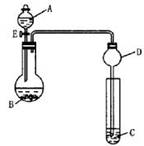
�� ��μ����װ�õ������� ��
�� ��AΪ30%H2O2��Һ��BΪMnO2��Cʢ�������ᣨH2S��������Һ������E��C�е�����Ϊ________________ ��B�з�����Ӧ�Ļ�ѧ����ʽΪ_____________ ��
�� ��AΪŨ���ᣬBΪKMnO4��C��ʢ��KI������Һ������E��C�е�������___________________ ��B�з�����Ӧ�����ӷ���ʽΪ_________________ ��
�� ͼ��Dװ����ʵ���е�������____________________________ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com