
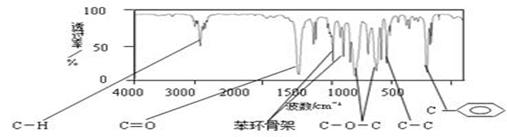
ij��A�Ļ�ѧʽΪC9H10O2����A������ֻ����1��������������ֻ��һ��ȡ�������ֲ��A��1H-NMR��ͼ��5���壬�����֮��Ϊ1�U2�U2�U2�U3�����ú�������ǿɳ�������л��������е�ijЩ���ţ��ֲ��A���ӵĺ����������ͼ��
 |
�Իش��������⡣
��1��A�Ľṹ��ʽ��Ϊ ����д������ͬ������������л���Ľṹ
��ʽ�� �� ��
��2��A�ķ�����ͬ���칹���ж��֣���д�����в����������ڷ������B�Ľṹ��ʽ_______��
��3����֪C��A��ͬ���칹�壬�����к���һ����������FeCl3��Һ����ɫ��������ֻ��������λȡ�����ķ���ȩ��C�Ľṹ��ʽ_______________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������и��������У�����֮��ͨ��һ����Ӧ����ʵ����ͼ��ʾ
 ת������ (����)
ת������ (����)
| ���� ���� ѡ�� | a | b | c |
| A | Al | AlCl3 | Al(OH)3 |
| B | HNO3 | NO | NO2 |
| C | Si | SiO2 | H2SiO3 |
| D | CH2===CH2 | CH3CH2OH | CH3CHO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���н�����ʵ�Ļ�ѧ����ʽ�����ӷ���ʽ����ȷ���� (����)
A����������������ʴ��2Fe��O2��2H2O===2Fe(OH)2
B��SO2ʹ��ɫʯ����Һ���ɫ��SO2��H2O===2H����SO
C������NaOH��Һ��ȥ���������������Ĥ��Al2O3��2OH����3H2O===2[Al(OH)4]��
D��84����Һ�ͽ������ʹ�û�����ж����壺Cl����ClO����2H��===Cl2����H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʽΪC4H7Cl���ȴ�ϩ����ͬ���칹�壨��˳���칹������ĿΪ
A��8 B��9 C��10 D��11
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ȩ������ͱ�ȩ��ɵĻ�����У���Ԫ�ص�����������37%����̼Ԫ�ص���������Ϊ
A��27% B��28% C��54% D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������������ϡ���ᷴӦ���ų�NO���ʵ���������
A��FeO B. Fe2O3 C. FeSO4 D��Fe3O4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϳ����ú����ˮ����Na2S2O3•5H2O��ʵ���ҿ�������װ�ã���ȥ���ּӳ�������ģ�����ɹ��̡�

��ƿC�з�����Ӧ���£�
Na2S��aq��+H2O��l��+SO2��g��=Na2SO3��aq��+H2S��aq�� ��I��
2H2S��aq��+SO2��g��=3S��s��+2H2O��l�� ��II��
S��s��+Na2SO3��aq�� Na2S2O3��aq�� ��III��
Na2S2O3��aq�� ��III��
��1��������װ��ɺر����˻�������װ��B�еij���©����ע��Һ�����γ�һ��Һע���� ��������װ�����������á�װ��D��������
��װ��E��Ϊ ��Һ��
��2��Ϊ��߲�Ʒ���ȣ�Ӧʹ��ƿC��Na2S��Na2SO3ǡ����ȫ��Ӧ������ƿC��Na2S��Na2SO3���ʵ���֮��Ϊ ��
��3��װ��B������֮һ�ǹ۲�SO2���������ʣ����е�Һ�����ѡ�� ��
a������ˮ b .����Na2SO3��Һ
c.����NaHSO3��Һ d . ����NaHCO3��Һ
ʵ���У�ΪʹSO2����������ƿC�����õIJ����� ����֪��Ӧ��III����Խ���������ƿC�з�Ӧ�ﵽ�յ�������� ����Ӧ���ڿ��þƾ����ʵ�������ƿA��ʵ�����þƾ��Ƽ���ʱ����ʹ��ʯ�������������� ��
a .�ձ� b .������ c.�Թ� d .��ƿ
��4����Ӧ��ֹ����ƿC�е���Һ������Ũ����������Na2S2O3•5H2O�����п��ܺ���Na2SO3��Na2SO4�����ʡ����������Լ����ʵ�飬����Ʒ���Ƿ����Na2SO4����Ҫ˵��ʵ�����������ͽ��ۣ�
��
��֪Na2S2O3•5H2O�����ֽ⣺S2O32‾+2H+=S��+SO2��+H2O
��ѡ����Լ���ϡ���ᡢϡ���ᡢϡ���ᡢBaCl2��Һ��AgNO3��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���淴Ӧ��2NO2(g) 2NO(g)+O2(g)�������������ܱ������з�Ӧ���ﵽƽ��״̬�ı�־��
2NO(g)+O2(g)�������������ܱ������з�Ӧ���ﵽƽ��״̬�ı�־��
A����λʱ��������n mol O2��ͬʱ����n mol NO2
B����λʱ��������n mol O2��ͬʱ����2n mol NO
C�����������ܶȲ��ٸı��״̬
D������������ɫ���ٸı��״̬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��(H2NNH2)��һ�ָ���ȼ�ϣ��йػ�ѧ��Ӧ�������仯��ͼ��ʾ����֪����1mol��ѧ�������������kJ����N��NΪ942��O=OΪ500��N-NΪ154�������1molN-H�������������KJ����
A.194 B.391 C.526.7 D.658

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com