

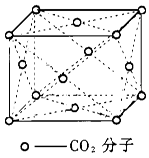



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
| A����Ͳ | B���ձ� | C������ƿ | D���Թ� |
|
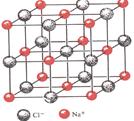
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A�����ʯ�����У�̼ԭ������C��C����֮��Ϊ1:2 |
B��720g C60���庬��NA����ͼ�о�����Ԫ |
| C�����Ӿ�����ÿ��������Χ��������6�����෴��ɵ����� |
| D��Cu�Ķѻ���ʽ�������������ܶѻ�����λ����8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A�������ֻ�ҶƬ���� | B������ɫ���� |
| C����������ģ�ߡ��� | D��������ͻ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ÿĦ̼ԭ����4 mol��ԭ���γɹ��ۼ� |
| B�����кܸߵ��۵㡢�е��Ӳ�� |
| C��Ӳ�Ⱥܴ�������ĥ���� |
| D������Һ����������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
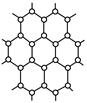
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
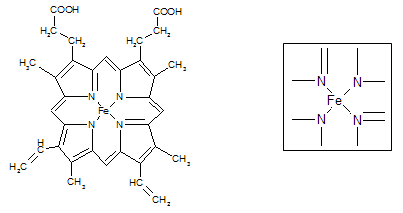
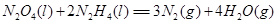
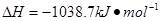
 ����___________mol��
����___________mol��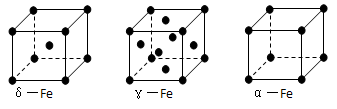
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com