
��12�֣�ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡһ���������Ͻ���100mLijŨ�ȵ������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4mol��L��1������������Һ����������������Һ�����(mL)������ij��������ʵ���(mol)�Ĺ�ϵ��ͼ��ʾ��C>0�����Իش��������⣺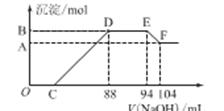
��1��д����Ӧ�����е����ӷ�Ӧ����ʽ��
OC�� ��
DE�� ��
EF��___________________________________ __________��
��2��������Һ�����ʵ���Ũ��Ϊ mol��L��1
��3��B��ֵΪ_________mol��C��ֵΪ mL��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣��������淢�����ڣ��ڸ��������γ�һ�����ܵ�������Fe3O4�����������ĥ����ʴ���ܡ���ԭ���ǣ�
����NaOH��Һ�У��������ܽ���NaNO2��Һ�У���ˮ֮�⣬���ɲ���A��C������CΪ���壬��ʹʪ��ĺ�ɫʯ����ֽ������
��A���ڹ�����NaNO2��Һ�м�����Ӧ������B��C��
��A��B����Һ�ܼ�����Ӧ������Fe3O4��
���о����֣�A��B����ɫ��Ӧ��Ϊ��ɫ���䵼����ʵ���ΪK2SO4�͡���������A��C��B��C�����ʵ���֮�Ⱦ�Ϊ31���ش��������⣺
4-1 д������ƽ��ѧ��Ӧ����ʽ��
4-2 ʵ���з����ʵ�����¶Ȼ�����NaNO2��Һ��Ũ������������Ĥ�����Ӵ�NaOH��ҺŨ�ȶ�Ĥ����Ӱ�첻����˵��ԭ��
4-3 ��������������COCl2����������ʱ��ϴ��������������Խ����ƻ���д���йصĻ�ѧ��Ӧ����ʽ��
4-4 ��һ�����β���D����B��Zn(NO3)2 ��Ӧ���ɣ�Ҳ������������������п���������Ƶ�Ϊԭ�ϵ�ˮ�Ⱥϳɷ�����ȷ��D�Ļ�ѧʽ�����ж������Ʊ�D�ķ�Ӧ�Ƿ�����������ԭ��Ӧ���˷����ò�ƷD�ܹ����ε�ԭ����ʲô��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com