
 ��Ҫ����գ�
��Ҫ����գ�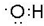 ��
�� ��
������ ��1���ǻ�Ϊ����ԭ���ţ���ԭ�������Ϊ7�����ӣ�
��2�����л���Ϊ��������������������ԭ��д�������ƣ�
��3��2-��-1��3-����ϩ������Ϊ1��3-����ϩ����2��C����1�������ݴ�д�������ʽ��
��4����ȩ��������Һ��Ӧ��������李�����������ˮ��
��5���ڲ���Ľṹ��ʽ������̼̼�������ɵõ���Ȳ���Ľṹ��ʽ��
��6����������ˮ������������Ҵ�������������Ӧԭ���������ǻ������⡱д����Ӧ�Ļ�ѧ����ʽ��
��� �⣺��1���ǻ��к���1��O-H����Ϊ����ԭ���ţ��ǻ��ĵ���ʽΪ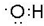 ��
��
�ʴ�Ϊ�� ��
��
��2����CH3CH2��2C��CH3��2���̼������5��C������Ϊ���飬��3��C����2������������Ϊ��3��3-�������飬
�ʴ�Ϊ��3��3-�������飻
��3��2-��-1��3-����ϩ�У�����Ϊ1��3-����ϩ������2��C�������ʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
��4����ȩ�к���ȩ�����ܹ���������Һ����������ԭ��Ӧ����Ӧ�Ļ�ѧ����ʽΪ��CH3CHO+2[Ag��NH3��2]OH$\stackrel{ˮԡ}{��}$CH3COONH4+2Ag��+3NH3+H2O��
�ʴ�Ϊ��CH3CHO+2[Ag��NH3��2]OH$\stackrel{ˮԡ}{��}$CH3COONH4+2Ag��+3NH3+H2O��
��5��ij����1��̼̼������Ȳ�����⻯��õ��ò���ڸò���Ľṹ��ʽ������̼̼�������ɵõ���Ȳ���Ľṹ��ʽΪ��CH��CH��CH3CH2��CH2CH��CH3��CH��CH3��CH3��
�ʴ�Ϊ��CH��CH��CH3CH2��CH2CH��CH3��CH��CH3��CH3��
��6��������������H218O��ϡ�����ֻ�ϲ����ȣ�18Oԭ�ӻ�ת���������е��ǻ�O����Ӧ�Ļ�ѧ����ʽΪ��CH3COOCH2CH3+H218O $?_{��}^{Ũ����}$CH3CO18OH+CH3CH2OH��
�ʴ�Ϊ��CH3COOCH2CH3+H218O $?_{��}^{Ũ����}$CH3CO18OH+CH3CH2OH��
���� ���⿼�����л���ṹ�����ʣ���Ŀ�Ѷ��еȣ��漰����ʽ���ṹ��ʽ���л���������������Ӧ��֪ʶ����ȷ�����л���ṹ������Ϊ���ؼ�������������ѧ�������Ӧ��������


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ԭ����������������ԭ�������������� | |
| B�� | ��ԭ�ӵ��Ӳ�������ԭ�ӵ��Ӳ����� | |
| C�� | 1mol�״������û����ɵ�������1mol�Ҵ������û����ɵ������� | |
| D�� | �����£�������ˮ��Ӧ�������������Ҳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
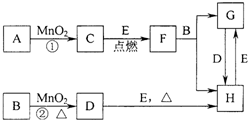 ��ͼ���漰�����ʾ�Ϊ��ѧ��ѧ�еij������ʣ�����C��D��EΪ���ʣ�����Ϊ�����FΪ�������ʣ����Ǵ�������ת����ϵ����Ӧ�����ɵ�ˮ����Ҫ���������ȥ��
��ͼ���漰�����ʾ�Ϊ��ѧ��ѧ�еij������ʣ�����C��D��EΪ���ʣ�����Ϊ�����FΪ�������ʣ����Ǵ�������ת����ϵ����Ӧ�����ɵ�ˮ����Ҫ���������ȥ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����� | ���� | ���� |
| A | �ֱ����Na2CO3��NaHCO3���� | �Թ��ڱھ���ˮ�� | �������ʾ����ȷֽ� |
| B | ����з�̪��NaOH��Һ�� ͨ��SO2 | ��Һ��ɫ��ȥ | SO2����Ư���� |
| C | ��I-����ɫ��Һ�еμ�����������ˮ���ٵμӵ�����Һ | ������ۺ���Һ�����ɫ | �����ԣ�Cl2��I2 |
| D | ��FeSO4��Һ���ȵ���KSCN��Һ���ٵμ�H2O2��Һ | ����H2O2����Һ���Ѫ��ɫ | Fe2+�������������л�ԭ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 11gT2O���еĵ�����Ϊ5NA | |
| B�� | �����£�0.2L 0.5mol•L-1NH4NO3��Һ�ĵ�ԭ����С��0.2NA | |
| C�� | ��4molHCl��Ũ�����������������̷�Ӧת�Ƶĵ�������ΪNA | |
| D�� | ��״���£�2.24L H2Sȫ������ˮ������Һ��HS-��S2-���Ӽ�֮��Ϊ0.1NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ�������£�Cl2���ڼױ��ı���������Ϸ���ȡ����Ӧ | |
| B�� | ����ͱ�ϩ�����ʵ�����1mol�����ȼ������3molH2O | |
| C�� | 1-������2-������һ�ȴ������ͬ | |
| D�� | ����屽����һ�ֿռ�ṹ��֤���������в����ڵ�˫������Ľṹ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��1����2����4����6�� | B�� | ��1����2����3����5�� | C�� | ��2����4����5����6�� | D�� | ��3����4����5����6�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���뷴Ӧ����������0.18mol | B�� | ��0.12mol H2SO4����ԭ | ||
| C�� | ��Ӧ����Һ����H2SO4ʣ�� | D�� | ����пƬΪ7.8g |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ʵ����� | ʵ������ |
| ���ɼУ�ͨ��һ��ʱ��CO2���رյ��ɼУ� | |
| ��Һ©����������Ũ���Ỻ��������ƿ�У��رջ����� | ���������� |
| ������ƿ����Ӧ��ʼ��ֹͣ���ȣ� | ��A���к���ɫ���������һ��ʱ���������ɫ��dz�� B����Һ����ɫ�� C����Һ��ɫ��dz�� �ڷ�Ӧֹͣ��A������ʣ�࣬��100mL����Һ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com