
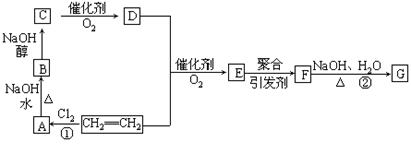
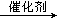 2C4H6O2��������ϩ����+2H2O
2C4H6O2��������ϩ����+2H2O �ṹ���Ƶ��л��ﲻ�ȶ�������������������
�ṹ���Ƶ��л��ﲻ�ȶ�������������������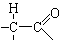
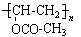 ����2���ӳɷ�Ӧ��ˮ�ⷴӦ��
����2���ӳɷ�Ӧ��ˮ�ⷴӦ��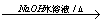 ClCH2CH2OH+HCl��ClCH2CH2OH+ NaOH
ClCH2CH2OH+HCl��ClCH2CH2OH+ NaOH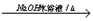 CH3CHO+NaCl+H2O��
CH3CHO+NaCl+H2O��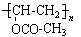 ���ԣ�1���У�DΪCH3COOH��FΪ
���ԣ�1���У�DΪCH3COOH��FΪ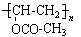 ����2���У��١��ڵķ�Ӧ�ֱ�ΪCH2=CH2+Cl2
����2���У��١��ڵķ�Ӧ�ֱ�ΪCH2=CH2+Cl2 ClCH2CH2Cl��
ClCH2CH2Cl��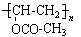
 (C2H4O)n+nCH3COOH�����䷴Ӧ���ͷֱ�Ϊ�ӳɷ�Ӧ��ˮ�ⷴӦ����3���У�A����B�ķ�Ӧ����ʽΪClCH2CH2Cl+H2O
(C2H4O)n+nCH3COOH�����䷴Ӧ���ͷֱ�Ϊ�ӳɷ�Ӧ��ˮ�ⷴӦ����3���У�A����B�ķ�Ӧ����ʽΪClCH2CH2Cl+H2O 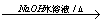 ClCH2CH2OH+HCl��B����C�ķ�Ӧ����ʽΪClCH2CH2OH+ NaOH
ClCH2CH2OH+HCl��B����C�ķ�Ӧ����ʽΪClCH2CH2OH+ NaOH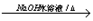 CH3CHO+NaCl+H2O��
CH3CHO+NaCl+H2O��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
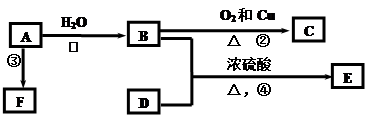
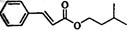 ����һ�����ϣ�һ�ֺϳ�·�����£�
����һ�����ϣ�һ�ֺϳ�·�����£�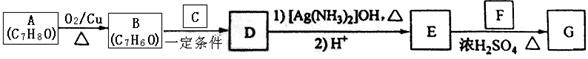
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
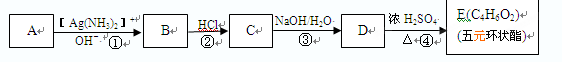 (1)X�ķ���ʽΪ ��
(1)X�ķ���ʽΪ ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ƶ���
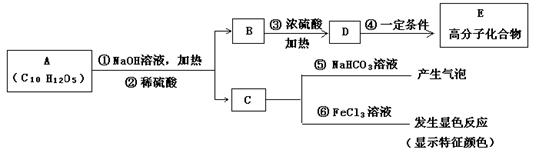




 ��֪B����Է�������Ϊ60��������ֻ��һ������C�Ľṹ�ɱ�ʾΪ��
��֪B����Է�������Ϊ60��������ֻ��һ������C�Ľṹ�ɱ�ʾΪ��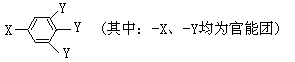
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
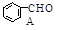 ��CH3CHO
��CH3CHO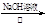
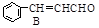 ��H2O
��H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
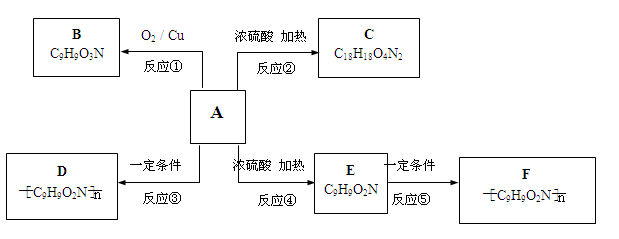
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com