
|
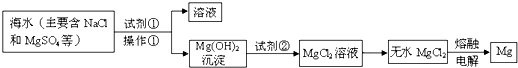
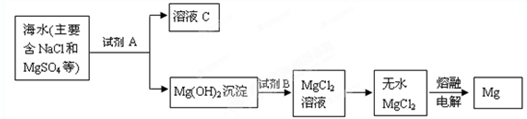
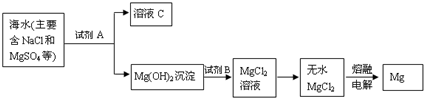
“Óŗ£Ė®ÖŠĢįČ”Ć¾µÄÖ÷ŅŖ²½Öč£»¢Ł°Ń±“æĒÉÕ³ÉÉśŹÆ»Ņ£»¢ŚŌŚŗ£Ė®ÖŠ¼ÓČėÉśŹÆ»Ņ£¬¹żĀĖ£¬Ļ“µÓ³ĮµķĪļ£»¢Ū½«³ĮµķĪļÓėŃĪĖį·“Ó¦£¬½į¾§”¢¹żĀĖ£»¢ÜŌŚĀČ»ÆĒāČČĘųĮ÷ÖŠ¼ÓČČ¾§Ģ壻¢Żµē½āÉĻŹöĖłµĆŃĪ£®ĻĀĮŠĖµ·Ø²»ÕżČ·µÄŹĒ | |
| [””””] | |
A£® |
ÉĻŹö±ä»Æ°üĄØĮĖ·Ö½ā·“Ó¦”¢»ÆŗĻ·“Ó¦”¢ø“·Ö½ā·“Ó¦ĄąŠĶ |
B£® |
±“æĒÉÕ³ÉÉśŹÆ»ŅŹōÓŚĪüČČ·“Ó¦ |
C£® |
ŌŚĀČ»ÆĒāČČĘųĮ÷ÖŠøÉŌļ¾§ĢåµÄÄæµÄŹĒĪŖĮĖŅÖÖĘŃōĄė×ÓµÄĖ®½ā |
D£® |
²½Öč¢ŻŅ²æÉŅŌ²ÉÓƵē½āøĆŃĪĖ®ČÜŅŗµÄ·½·Ø |

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

| ||
| ||
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢ÉĻŹö±ä»Æ°üĄØĮĖ·Ö½ā·“Ó¦”¢»ÆŗĻ·“Ó¦”¢ø“·Ö½ā·“Ó¦ĄąŠĶ | B”¢±“æĒÉÕ³ÉÉśŹÆ»ŅŹōÓŚ·ÅČČ·“Ó¦ | C”¢ŌŚĀČ»ÆĒāČČĘųĮ÷ÖŠøÉŌļ¾§ĢåµÄÄæµÄŹĒĪŖĮĖŅÖÖĘŃōĄė×ÓµÄĖ®½ā | D”¢²½Öč¢ŻŅ²æÉŅŌ²ÉÓƵē½āøĆŃĪĖ®ČÜŅŗµÄ·½·Ø |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A”¢ŗ£Ė®
| |||||||
B”¢ŗ£Ė®
| |||||||
C”¢ŗ£Ė®
| |||||||
D”¢ŗ£Ė®
|
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com