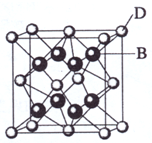
��2009?���ϣ���֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ����ͼ��
��ش�
��1��AԪ�ص�������
��
��
��
��2��B��Ԫ�ط�����
F
F
��C��Ԫ�ط�����
Cl
Cl
��B��A�γɵĻ������C ��A�γɵĻ�����е�ߣ���ԭ����
��������Ӽ����������Ȼ�����Ӽ�û�����
��������Ӽ����������Ȼ�����Ӽ�û�����
��3��E��Ԫ�����ڱ��е�
��
��
���ڣ���
VIIB
VIIB
���Ԫ�أ���Ԫ��������
��
��
������+2�����ӵĵ����Ų�ʽΪ
1s22s22p63s23p63d5
1s22s22p63s23p63d5
��
��4����ͼ�п��Կ�����D��B�γɵ����ӻ�����Ļ�ѧʽΪ
CaF2
CaF2
�������ӻ����ᄃ����ܶ�Ϊa g?cm
-3�����������
| 4��78g/mol |
| ag?cm-3��6.02��1023/mol |
| 4��78g/mol |
| ag?cm-3��6.02��1023/mol |
��ֻҪ���г���ʽ����

 ��2009?���ϣ���֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ����ͼ��
��2009?���ϣ���֪A��B��C��D��E����Ԫ�����ڱ���ǰ36�ŵ�Ԫ�أ����ǵ�ԭ��������������A������4��Ԫ�ؼȲ���ͬһ�����ֲ���ͬһ���壮B��C��ͬһ���壬D��E��ͬһ���ڣ���֪E�����ڱ���1-18���еĵ�7��Ԫ�أ�D��ԭ��������EС5��D��B���γ����ӻ������侧���ṹ����ͼ��

