
£¨±¾̀â¹²10·Ö£©°×Áס¢º́Á×ÊÇÁ×µÄÁ½ÖÖͬËØ̉́¹¹̀壬ÔÚ¿ƠÆøÖĐȼÉƠµĂµ½Á×µÄÑơ»¯Î¿ƠÆø²»×ăʱÉú³ÉP4O6£¬¿ƠÆø³ä×ăÊÇÉú³ÉP4O10¡£
£¨1£©̉ÑÖª298Kʱ°×Áס¢º́Á×ÍêȫȼÉƠµÄÈÈ»¯Ñ§·½³̀ʽ·Ö±đΪ£º
P4(s,°×Á×)+5O2(g)=P4O10(s) ¦¤H1=" -2983.2" kJ?mol-1,
P(s£¬º́Á×)+ 5/4O2(g)="1/4" P4O10(s) ¦¤H2=" -738.5" kJ?mol-1
Ộ¸ĂζÈÏ°×Á×ת»¯Îªº́Á×µÄÈÈ»¯Ñ§·½³̀ʽΪ ¡£
£¨2£©̉ÑÖª298Kʱ°×Áײ»ÍêȫȼÉƠµÄÈÈ»¯Ñ§·½³̀ʽΪP4(s,°×Á×)+3O2(g)=P4O6(s) ¦¤H= -1638kJ?mol-1¡£ÔÚijĂܱƠÈƯÆ÷ÖĐ¼ÓÈë62g°×Á׺Í50.4LÑơÆø£¨±ê×¼×´¿öÏ£©£¬¿ØÖÆ̀ơ¼₫ʹ֮ǡºĂÍêÈ«·´Ó¦¡£ỘËùµĂµ½µÄP4O10ºÍP4O6µÄÎïÖʵÄÁ¿Ö®±ÈΪ £¬·´Ó¦¹ư³̀ÖĐ·Å³öµÄÈÈÁ¿ÎªÎª ¡£
£¨3£©̉ÑÖª°×Á׺ÍPCl3µÄ·Ö×ӽṹÈçͼËùʾ£¬ÏÖ̀ṩ̉ÔϵĻ¯Ñ§¼üµÄ¼üÄÜ£¨KJ£¯mol£©:P-P 198£¬Cl-Cl 243£¬P-Cl 331¡£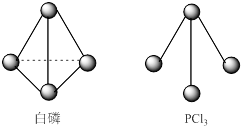
Ộ·´Ó¦P4(s,°×Á×)+6Cl2(g)=4PCl3(s)µÄ·´Ó¦ÈȦ¤H = ¡£

| Ä꼶 | ¸ßÖĐ¿Î³̀ | Ä꼶 | ³ơÖĐ¿Î³̀ |
| ¸ß̉» | ¸ß̉»Ăâ·Ñ¿Î³̀ÍƼö£¡ | ³ở» | ³ở»Ăâ·Ñ¿Î³̀ÍƼö£¡ |
| ¸ß¶₫ | ¸ß¶₫Ăâ·Ñ¿Î³̀ÍƼö£¡ | ³ơ¶₫ | ³ơ¶₫Ăâ·Ñ¿Î³̀ÍƼö£¡ |
| ¸ßÈư | ¸ßÈưĂâ·Ñ¿Î³̀ÍƼö£¡ | ³ơÈư | ³ơÈưĂâ·Ñ¿Î³̀ÍƼö£¡ |
¿ÆÄ¿£º¸ßÖĐ»¯Ñ§ À´Ô´£º2014½́½Î÷Ê¡ÄϲưÊи߶₫µÚ̉»´ÎÔ¿¼»¯Ñ§ÊÔ¾í£¨½âÎö°æ£© ̀âĐÍ£º¼ÆËằâ
£¨±¾̀â¹²10·Ö£©°×Áס¢º́Á×ÊÇÁ×µÄÁ½ÖÖͬËØ̉́¹¹̀壬ÔÚ¿ƠÆøÖĐȼÉƠµĂµ½Á×µÄÑơ»¯Î¿ƠÆø²»×ăʱÉú³ÉP4O6£¬¿ƠÆø³ä×ăÊÇÉú³ÉP4O10¡£
£¨1£©̉ÑÖª298Kʱ°×Áס¢º́Á×ÍêȫȼÉƠµÄÈÈ»¯Ñ§·½³̀ʽ·Ö±đΪ£º
P4(s,°×Á×)+5O2(g)=P4O10(s) ¦¤H1= -2983.2 kJ•mol-1,
P(s£¬º́Á×)+ 5/4O2(g)=1/4 P4O10(s) ¦¤H2= -738.5 kJ•mol-1
Ộ¸ĂζÈÏ°×Á×ת»¯Îªº́Á×µÄÈÈ»¯Ñ§·½³̀ʽΪ ¡£
£¨2£©̉ÑÖª298Kʱ°×Áײ»ÍêȫȼÉƠµÄÈÈ»¯Ñ§·½³̀ʽΪP4(s,°×Á×)+3O2(g)=P4O6(s) ¦¤H= -1638kJ•mol-1¡£ÔÚijĂܱƠÈƯÆ÷ÖĐ¼ÓÈë62g°×Á׺Í50.4LÑơÆø£¨±ê×¼×´¿öÏ£©£¬¿ØÖÆ̀ơ¼₫ʹ֮ǡºĂÍêÈ«·´Ó¦¡£ỘËùµĂµ½µÄP4O10ºÍP4O6µÄÎïÖʵÄÁ¿Ö®±ÈΪ £¬·´Ó¦¹ư³̀ÖĐ·Å³öµÄÈÈÁ¿ÎªÎª ¡£
£¨3£©̉ÑÖª°×Á׺ÍPCl3µÄ·Ö×ӽṹÈçͼËùʾ£¬ÏÖ̀ṩ̉ÔϵĻ¯Ñ§¼üµÄ¼üÄÜ£¨KJ£¯mol£©:P-P 198£¬Cl-Cl 243£¬P-Cl 331¡£

Ộ·´Ó¦P4(s,°×Á×)+6Cl2(g)=4PCl3(s)µÄ·´Ó¦ÈȦ¤H = ¡£
²é¿´´đ°¸ºÍ½âÎö>>
°Ù¶ÈÖÂĐÅ - Á·Ï°²áÁбí - ÊỒâÁбí
º₫±±Ê¡»¥ÁªÍøÎ¥·¨ºÍ²»Á¼ĐÅÏ¢¾Ù±¨Æ½̀¨ | ÍøÉÏÓĐº¦ĐÅÏ¢¾Ù±¨×¨Çø | µçĐÅƠ©Æ¾Ù±¨×¨Çø | ÉæÀúÊ·ĐéÎ̃Ö÷̉åÓĐº¦ĐÅÏ¢¾Ù±¨×¨Çø | ÉæÆóÇÖȨ¾Ù±¨×¨Çø
Î¥·¨ºÍ²»Á¼ĐÅÏ¢¾Ù±¨µç»°£º027-86699610 ¾Ù±¨ÓÊÏ䣺58377363@163.com