
���� ��1��������Һ������ԭ����֪���ù���������Һ��һ�㲽��Ϊ���㡢�������ܽ�ת�ơ�ϴ��ת�ơ�����ҡ�ȣ�
��2������m=nM=cVM������Ҫ�������Ƶ�������
��3��һ���ݻ�������ƿֻ������һ���������Һ������0.1mol•L-1��NaOH��Һ0.5L��������ѡ��500ml������ƿ�����ݾ���IJ������裬ѡ�������������
��4��NaOHӦ����С�ձ��г�����
��5������ƿ��ʹ��ǰҪ�Ȳ�©��
��6������������������ʵ����ʵ�������Һ�����Ӱ�죬����c=$\frac{n}{v}$�ж���
��δ��������ʱ����������Һת��������ƿ���ݣ���Һ������������ȴ����Һ���С��500ml��
�ڸǵ�תҡ�Ⱥ���������ڿ̶��ߣ�һ������Һ����ƿ����ƿ��֮�䣬�ֵμ�����ˮ���̶ȣ�ʹ��Һ�������500ml��
��7����ˮ����������ƿ�̶��ߣ��˴�����ʧ�ܣ���Ҫ�������ƣ�
��8���������������ʵ����ʵ�������Һ�������Ӱ�죬����c=$\frac{n}{V}$�жϣ�
��� �⣺��1��������Һ������ԭ����֪���ù���������Һ��һ�㲽��Ϊ���㡢�������ܽ�ת�ơ�ϴ��ת�ơ�����ҡ�ȣ�
�ʴ�Ϊ�����㡢�������ܽ�ת�ơ�ϴ��ת�ơ�����ҡ�ȣ�
��2������0.1mol•L-1��NaOH��Һ450ml��Ӧѡ��500mL����ƿ����Ҫ�������Ƶ�����Ϊ0.1mol•L-1��0.5L��40g/mol=20g��
�ʴ�Ϊ��20��
��3������0.1mol•L-1��NaOH��Һ450ml��������ѡ��500ml������ƿ����������ҩ��ȡNaOH���ܽ���Ҫ���ձ������������裬ת�ƹ������ò���������������ý�ͷ�ιܶ��ݣ��ʻ�������Ϊ�ձ���ҩ�ס�����������ͷ�ιܣ�
�ʴ�Ϊ��500ml���ձ���ҩ�ס�����������ͷ�ιܣ�
��4��NaOH������ˮ�ԣ���������ж�����̼��Ӧ������ʱӦ������С������ĽӴ������Ӧ����С�ձ��г�����
��ѡ�ۣ�
��5������ƿ�л���������ʹ��ǰҪ�ȼ���Ƿ�©ˮ��
�ʴ�Ϊ������Ƿ�©ˮ��
��6����δ��������ʱ����������Һת��������ƿ���ݣ���Һ�����������ָ������º���Һ���С��500ml��������ҺŨ��ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ���ָ������º���Һ���С��500ml��
�ڸǵ�תҡ�Ⱥ���������ڿ̶��ߣ�һ������Һ����ƿ����ƿ��֮�䣬�ֵμ�����ˮ���̶ȣ�ʹ��Һ�������500ml����ҺŨ��ƫ�ͣ�
�ʴ�Ϊ��ƫ�ͣ���Һ�������500ml��
��7��������ʱ�����ˮ��������������ƿ�̶��ߣ��˴�����ʧ�ܣ����Ѿ����Ƶ�������Һ�������ã�Ȼ���������ƣ�
�ʴ�Ϊ���������ƣ��������ã�
��8����û��ϴ���ձ��Ͳ�������������Һ���������Ƶ����ʵ�����С������c=$\frac{n}{V}$��֪�����Ƶ���ҺŨ��ƫ�ͣ��ʢٴ���
�ڳ���NaOH��ʱ��̫���������������Ƴ��⡢���ֱ��ʣ����Ƶ���Һ���������Ƶ����ʵ���ƫС������c=$\frac{n}{V}$��֪�����Ƶ���ҺŨ��ƫ�ͣ��ʢڴ���
�۶���ʱ���ӿ̶ȣ�������Һ�����ƫС������c=$\frac{n}{V}$��֪�����Ƶ���ҺŨ��ƫ�ߣ��ʢ���ȷ��
��NaOH��Һδ��ȴ�����¾�ת�Ƶ�����ƿ��������Һ�����ƫС������c=$\frac{n}{V}$��֪�����Ƶ���ҺŨ��ƫ�ߣ��ʢ���ȷ��
������ƿ�����������������ˮ�����ڶ���ʱ����Ҫ��������ˮ�����Բ�Ӱ�����ƽ�����ʢݴ���
��ѡ�ۢܣ�
���� ���⿼��������һ�����ʵ���Ũ�ȵ���Һ�ķ�������Ŀ�Ѷ��еȣ����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ��������������ѧ���淶ʵ�����������������ѵ�������������ע����ȷ�������ķ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  �㵹Һ�� | B�� |  ��ȼ�ƾ��� | C�� |  ϴ���Թ� | D�� |  ϡ��Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ����ƿ����û�к�� | B�� | ����ʱ�۲�Һ�温�� | ||
| C�� | ����ʱ�۲�Һ������ | D�� | ���ݺ�ҡ��ʱ������Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
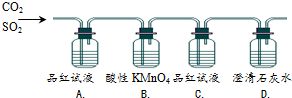
| ��� | ʵ������ | ʵ��Ŀ�� |
| A�� | Ʒ����ɫ | ֤�������������SO2 |
| B�� | KMnO4��ɫ | ֤��SO2�л�ԭ�� |
| C�� | Ʒ�첻��ɫ | ֤������C��������û��SO2 |
| D�� | ʯ��ˮ����� | ֤�������������CO2 |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������������ϡ���Fe��OH��2+2H+=Fe2++2H2O | |
| B�� | NH4Al��SO4��2��Һ�����NaOH��Һ��Ӧ��Al3++4OH-=AlO2-+2H2O | |
| C�� | Na2SiO3��Һ��ϡ�����ϣ�Na2SiO3+2H+=2Na++H2SiO3�� | |
| D�� | Ca��HCO3��2��Һ��Ca��OH��2��Һ��ϣ�Ca2++HCO3-+OH-=CaCO3��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
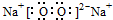 ��
��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com