
��ҵ�Ƶõĵ�����(AlN)��Ʒ�г���������Al4C3��Al2O3��C�����ʡ�ijͬѧ���������ʵ��ֱ�ⶨ������(AlN)��Ʒ��AlN��Al4C3����������(����NH3��ǿ������Һ�е��ܽ�)��
��1��ʵ��ԭ��
��Al4C3�����ᷴӦ������CH4��
��AlN����ǿ�������Σ�����ǿ�����ɰ�������д��AlN��NaOH��Һ��Ӧ�Ļ�ѧ����ʽ ��
��2��ʵ��װ��(��ͼ��ʾ)

��3��ʵ�����
������ʵ��װ�ã�����װ�õ������ԡ��Ƶ�Dװ�õ�����Ϊyg���ζ��ܵĶ���ΪamL��
�ڳ�ȡxg AlN��Ʒ������ƿ�У����ý������رջ��� ������ ��ͨ����Һ©������ϡ���ᣬ����ƿ�����ʳ�ַ�Ӧ��
�۴���Ӧ������ȫ�رջ��� ������ ��ͨ����Һ©���������
(�ѧʽ)������ƿ�����ʳ�ַ�Ӧ��
�� (����ò�Ӧ���еIJ���)��
�ݼ�¼�ζ��ܵĶ���ΪbmL���Ƶ�Dװ�õ�����Ϊzg��
��4�����ݷ���
��AlN����������Ϊ ��
������ȡ�ζ�������������ʱ��Һ������ҵͣ��������������� (�ƫ����ƫС������Ӱ�족)��
��Al4C3����������Ϊ (��ʵ�������µ�����Ħ�����ΪVm)��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijѧ��ȡ9 g��������ˮ��Ϊ�ⶨ���۵�ˮ���ʣ���ʵ���������£�
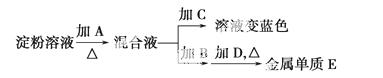
(1)���������Լ�Ϊ��A________��B________��C________��D________��
(2)����B��Һ�ܷ�õ�E________(��ܡ����ܡ�)������������________________
___________________________________________________________________________��
(2) ������2.16 g��������ʱ�����۵�ˮ����Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
L�����һ���л��������������ɭ���ۺ�֢�����ƣ���ṹ��ʽ����ͼ��ʾ������ҩ��������ǻ��ڻ��2000��ŵ��������ѧҽѧ���ͻ��2001��ŵ������ѧ�����о��ɹ������й���L��͵��ᡢ���Ե�������ȷ����(����)
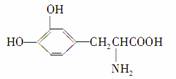
A����û�����ԣ�Ҳû�м���
B���Ⱦ������ԣ�Ҳ���м���
C��ֻ�����ԣ�û�м���
D��ֻ�м��ԣ�û������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л��������㴼��������ʳ���㾫����ṹ��ͼ��ʾ��
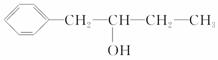
(1)�㴼�ķ���ʽΪ________�������ܷ������л���Ӧ������________(�����)��
��ȡ����Ӧ���ڼӳɷ�Ӧ������ȥ��Ӧ���ܾۺϷ�Ӧ
��������Ӧ����ˮ�ⷴӦ
(2)�л����(C13H18O2)��һ�����ϣ���ϳ�·����ͼ��ʾ�����м���Է�������Ϊ88�����ĺ˴Ź�����������3��壬��Ϊ�㴼��ͬϵ�

��֪��R��CH===CH2 R��CH2CH2OH
R��CH2CH2OH
��ش��������⣺
��A��ϵͳ��������Ϊ_________________________________________________��
��д��C������Cu(OH)2����Һ��Ӧ�Ļ�ѧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
�۱�������������������һ�������£� 1 mol D���Ժ�2 mol H2��Ӧ�����ң�D���Է���������Ӧ����D�Ľṹ��ʽΪ_________________________________________��
�ܼ����ҷ�Ӧ�Ļ�ѧ����ʽΪ____________________________________________
________________________________________________________________________��
�ݼ�ͬ���칹���к��С���COO�����ṹ�Ĺ���________�֡�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������Ԫ��X��Y��Z��W��Ԫ�����ڱ��е�λ�ù�ϵ����ͼ���������ۺ�������
 A����X��Y��Z��W��ֻ��һ��Ϊ����Ԫ�أ���Wһ��Ϊ��Ԫ��
A����X��Y��Z��W��ֻ��һ��Ϊ����Ԫ�أ���Wһ��Ϊ��Ԫ��
B. ��W�ĺ˵������Y����������W������������뵼�����
C����Z�ĺ˵������Y����������X���⻯��ˮ��Һ������
D. ��Y��W�ļ������Ӷ����ƻ�ˮ�ĵ��룬��Z�ļ�������Ҳһ�����ƻ�ˮ�ĵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д�������л���Ľṹ��ʽ������Υ��ϵͳ�����������Ծ�����
(1)3,5��������
____________________����ȷ���ƣ�____________��
(2)3,3,4,4�ļ�2�һ�����
____________________����ȷ���ƣ�____________��
(3)4,4,5,5�ļ�3��������
____________________����ȷ���ƣ�____________��
(4)2,3,4,5�ļ�3�һ�5��������
____________________����ȷ���ƣ�____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��HNCO�������ᣬ��ṹ��H��N��C��O���ܺ�NO2��Ӧ����N2��CO2��H2O�������й�������Ӧ����������ȷ����
A. HNCO�еĵ�Ԫ�ر����� B. NO2�еĵ�Ԫ�ر���ԭ
C.ÿ����1mol H2Oת��6mol���� D. CO2����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ�����ӵ�������ֵ�����������������( )
A�����³�ѹ�£�8.8g CO2��N2O��������������ĵ�����Ϊ4.4NA
B��5.6 g����500 mL 2 mol·L-1���ᷴӦ��ת�Ƶĵ�����Ϊ0.2NA
C�����³�ѹ�£�0.4 mol Na2O2������H2O��Ӧ��������0.2 mol O2��ת�Ƶ��ӵ���ĿΪ0.4NA
D. V La mol·L-1���Ȼ�����Һ�У���Fe3+����ĿΪ6.02��1023����Cl-����Ŀ����3��6.02��1023
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com