
��ҵ����������豸��Ϊ���֣�һ�Ƿ���¯�����ǽӴ��ҡ��������������ڷ���¯�����ջ��������ɶ��������ڽӴ������д��������¶��������һ����������ϣ�������������������������������ʱ������98.3%��Ũ�������գ�ʹ��������������ˮ�����γ����ᡣ
�����װ���Ƿ��չ�ҵ���Ʊ�����Ĺ���������Ƴ����ģ�����̽����ҵ��Ϊ�β���98.3%��Ũ����������������

��ش���������:
��1�� д������¯�����ջ�����ķ�Ӧ����ʽ ��
��2�� ��ͼ�е��ҡ����ֱ��൱�ڹ�ҵ����ȡ����װ���е� �� ��
��3�� ���е�����Ϊ �����е�����Ϊ ��
��4����ͼ��ѹǿ��SO2 ƽ��ת���ʵ�Ӱ��

��SO2 ת��ΪSO3�ķ�Ӧ������ѹǿ��ʹת���� ��֮����ͨ�����ó�ѹ��������Ϊ ��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
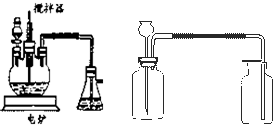 ����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�
����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�
| ||
| ||
| ||
| ||

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
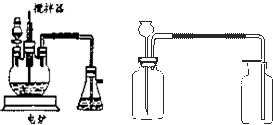 ����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�
����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com