
�������ֳƻ�������Ҫ�ɷ�FeS2�����ʲ�����Ԫ�أ����ǵؿ��зֲ������������dz�Ƶ���ɫ�������Ľ�������������Ϊ�ǻƽ𣬹��ֳơ����˽𡱡�
��ҵ����������Ҫ���ڽӴ����������ᣬ�䷴Ӧԭ��Ϊ��
��FeS2��O2�����·�Ӧ����SO2��
��SO2�ڴ�����������O2��Ӧ����SO3��
��SO3��H2O��Ӧ����H2SO4��
��1����1.00�� SO2��O2�Ļ�������к�SO2 0.40�֣���һ�������·�����Ӧ�ڣ���80%��SO2����ת������Ӧ����������SO3������������_________��
��2������80�������������ᣬ���������100��98%�����ᡣ����Ӧ��������Ԫ�ص���ʧ��Ϊ5%������������FeS2����������Ϊ__________________��
��3����Ũ�����м����ܽ�SO3�����γɵ�Һ��Ʒ������ᣬ��Ũ��ͨ���������SO3��������ʾ����20%�ķ������ἴ��ʾ�������к���20%��SO3������1L��SO330%�ķ������ᣨ�ܶ�Ϊ1.95g/cm3����Ҫ����ϡ�ͳ���������Ϊ95%��Ũ���ᣬ���ˮԼ���٣���д��������̣�
��4����֪��850�桫900��ʱ�������������������գ����ܷ������з�Ӧ��
��3FeS2��8O2��Fe3O4��6SO2
��4FeS2��11O2��2Fe2O3��8SO2
ΪʹFeS2������ȫ����Fe2O3����ҵ��ʹ�ù�������������������20%ʱ��������¯����SO2�������������д��������̣�
57����480 g������FeS2����������ȫ��Ӧ�������ù�����n��Fe����n��O����4��a����ʱ��������b mol����д��b��a�Ĺ�ϵʽ_______________________��
��1��0.4��40%40%
��2��78.9%
��3��241.2g
��4��78.4%
57��b��0.5a��8
���������������1��2SO2+O2 2SO3��n��SO2������n��SO3��. ������Ӧ��SO2����m��SO2��= 0.40�֡�80%=0.32�֣��������SO3������Ϊ80/64��0.32��=0.4�֡��ʷ�Ӧ����������SO3������������0.4��1��100%=0.4��40%��
2SO3��n��SO2������n��SO3��. ������Ӧ��SO2����m��SO2��= 0.40�֡�80%=0.32�֣��������SO3������Ϊ80/64��0.32��=0.4�֡��ʷ�Ӧ����������SO3������������0.4��1��100%=0.4��40%��
��2������Ԫ���غ��֪��ϵʽΪFeS2����2SO2����2SO3����2H2SO4��1mol FeS2����ȡ2mol H2SO4.��120��FeS2����ȫת������ȡ196�ִ��������ᡣ�������������FeS2����������ΪX������б���ʽ120:�� 2��98�� =80��X��1-5%��:�� 100��98%��.���X=78.9%��
��3������1L��SO330%�ķ������ᣨ�ܶ�Ϊ1.95g/cm3����Ҫ����ϡ�ͳ���������Ϊ95%��Ũ���ᣬ���ˮԼ���٣�m����Һ��= 1.95g/cm3��1000ml ="1950g" .m��SO3��= 1.95g/cm3��1000ml��30%=1950g="585" g.��SO3��������������Ϊ585 g��98/80=716.6g. m��H2SO4��= 1.95g/cm3��1000ml����1-30%��=1365g����1L��SO330%�ķ������ᣨ�ܶ�Ϊ1.95g/cm3����Ҫ����ϡ�ͳ���������Ϊ95%��Ũ���ᣬ���ˮ����ΪY����1950g+Y�� �� 95%=716.6g+1365g.���Y=241.2g��
��4��4FeS2��11O2 2Fe2O3��8SO2 ���跴Ӧ����8Ħ����SO2������������Ҫ11Ħ����O2��������������20%������ʵ��ͨ������������ʵ���Ϊ11��120%mol =13.2mol.����������Ϊ2.2mol.��ͬ��ͬѹ�£�����������˵���������Ⱦ������ǵ����ʵ����ıȡ�������¯����SO2���������8�£�8+2.2����100%=78.4%��
2Fe2O3��8SO2 ���跴Ӧ����8Ħ����SO2������������Ҫ11Ħ����O2��������������20%������ʵ��ͨ������������ʵ���Ϊ11��120%mol =13.2mol.����������Ϊ2.2mol.��ͬ��ͬѹ�£�����������˵���������Ⱦ������ǵ����ʵ����ıȡ�������¯����SO2���������8�£�8+2.2����100%=78.4%��
57����FeS2��n��Fe��:n��S��=1:2��480g FeS2�����ʵ���Ϊ480g��120g/mol=4mol.n��SO2��=8mol.n��Fe��=4mol�������ù�����n��Fe����n��O����4��a�� n��Fe��=4mol�����ڹ����е���ԭ�ӵ����ʵ���Ϊamol.�������Ĺ����й����ĵ����������ʵ���Ϊb��0.5a��8��
���㣺�����й��������������̵ķ�Ӧԭ����������Ʒ������ʵĺ����ȼ����֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����������������ᷢչ�����ʻ�������ͬ�IJ��Ϲ��ܸ�����ͬ�����¶����йز��ϵ����ݣ��Ը����������ݻش����⡣
(1)������δ����Դ���ѡ��֮һ�����ܵ������漰��Ĵ��桢���˺�ʹ�á�����Ͻ��ǽ����Ĵ�����������Ҫ���ϡ�����Ͻ�����ߴ������������Ͻ�(LaNi5)����֪LaNi5(s)��3H2(g)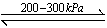 LaNi5H6(s)��H����31.77 kJ��mol��1��
LaNi5H6(s)��H����31.77 kJ��mol��1��
�����Ͻ���۵���硢�����۵�________(��ߡ����͡���������֮�䡱)�����ݷ�Ӧԭ�����γ�LaNi5H6�Ļ�ѧ��Ӧ������________�������������������Ƴ�һ��������Խ�Ĵ��������Դ���������Ͻ����������������Ϊ�ý���������________(����š������š�)����������___________________________________________________��
(2)���ǽ����������ճ������в���ȱ�ٵ����ʣ����������и�ǿ�ȡ����¡���ʴ���ص㡣Si3N4����һ����Ҫ�ľ�ϸ�մɣ��ϳɵ�����ķ���֮һΪ��
3SiO2��6C��2N2 Si3N4��6CO��Si3N4����________���壬��������Ӧ��������Ϊ________��
Si3N4��6CO��Si3N4����________���壬��������Ӧ��������Ϊ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������Ҫ�Ļ���ԭ�ϡ�
��1����������ˮ�õ���ˮ����ˮ�д������з�Ӧ��Cl2��H2O??H����Cl����HClO����ƽ�ⳣ������ʽΪK��________��
��2����ҵ�ϳ�����ʯ�Һ�������Ӧ��ȡƯ�ۣ���Ӧ�Ļ�ѧ����ʽ��____________________________����������ͼ��ʾ������Ҫ�豸���Ȼ����������ϵ��·��IJ㡣������3%��6%ˮ�ֵ���ʯ�Ҵ������������룬������������ײ�ͨ�룬�������ϵ�Ŀ����__________________________���������Ȼ������ݳ�����ķ�����________��
��3��ij����С����ʵ�����ý�Ũ��KOH��Һֱ�������������о����ַ�Ӧ����һ��ʱ���ʼ����KClO3�������࣬����KClO3�����ӷ���ʽ��____________________________________����ԭ�������__________________���ɴ˿�֪��2�����Ȼ������Ϊ�IJ���Ϊ�˼������������Ƹ���Ӧ�ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪A��B��C��D��E��F�Ǻ���ͬһ��Ԫ�صĻ��������F��ʹ��ɫʪ��ʯ����ֽ����ɫ������֮���ܷ������·�Ӧ��
�� A+H2O �� B+C �� C+F �� D �� D+NaOH �� F+E+H2O
��1��д�����ǵĻ�ѧʽ��D_______________��F_______________��
��2��д���ٷ�Ӧ�Ļ�ѧ����ʽ��_______________________________
��3��д����Ӧ�۵����ӷ���ʽ��___________________________��
��4����ҵ����C�Ĺ�����������һ����Ӧ����F������������B��H2O��д���ò���Ӧ�Ļ�ѧ����ʽ��________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
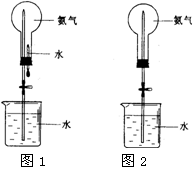
ʵ������ͼI��ʾ��װ����ȡ����İ�����
��1��A����Ӧ�Ļ�ѧ����ʽΪ ��
��2��Ϊ�ռ�������İ���������CӦ�� ������ţ���
��3��װ��B��ʢװ���Լ��������� ��
��4����ˮ���м���ˮ������̪�����������������Թܵ����ڷ���ˮ���У���ͼ����ʾ������Ĵָ���ƿ��Թܿڣ��ɹ۲쵽������Ϊ_______________ ��
��5����D��E��F���������ռ������У��������ռ�NO����________����д��ţ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��Ȫ��һ�ֳ�������Ȼ�����������ԭ���Ǵ���ѹǿ���ȡ�����������Ȫʵ�飨ͼ�мг�װ�þ�����ȥ����
��1��д��ʵ������ȡ�����Ļ�ѧ����ʽ�� _________ ��
��2���ռ�����Ӧʹ�� ����
��3��Ҫ�õ�����İ�����ѡ������ ���������
| A��Ũ���� | B����ʯ�� | C��NaOH���� | D��P2O5���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ô��Թ��ռ�һ�Թ�NO2���壬������ʢˮ��ˮ����Թ��ڲ���������Ϊ �������Թ���ͨ�������������Թ����ֲ���������Ϊ ���������������ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʯ��������Ṥҵ��β��(��NO��NO2)��Ӧ�����ܾ���β�������ܻ��Ӧ�ù㷺��Ca(NO2)2���䲿�ֹ����������£�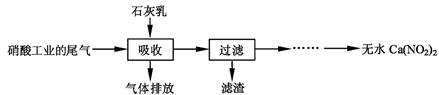
��ش��������⣺
��1��һ�������£�NO��NO2�������з�Ӧ��NO(g)��NO2(g)  N2O3(g)����ƽ�ⳣ������ʽΪK= ��
N2O3(g)����ƽ�ⳣ������ʽΪK= ��
��2�����������в�����Һ�����Ӵ�����(β�����������ײ����룬ʯ�������������������)����Ŀ���� ��������ѭ�����ã���������Ҫ�ɷ��� (�ѧʽ)��
��3���ù��������NO��NO2���ʵ���֮�Ƚӽ�1�U1����n(NO)��n(NO2)��1�U1,��ᵼ�� ����n(NO)��n(NO2)��1�U1,��ᵼ�� ��
��4����������Һ�豣�������ԣ���������Һ��Ca(NO2)2�ᷢ���ֽ⣬����֮һ��NO���䷴Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��13�֣�ֱ���ŷŵ���������γ����ꡢ����������ԭ�����������շ�ʦ���õĴ���������
��1���û�ѧ����ʽ��ʾNO2�γ�����Ĺ���____________________________
��2������NH3��CH4�������ȥ�����еĵ������
��NH3�ĵ���ʽ
�����ȶ��� NH3 CH4�������=��<����
����֪��CH4(g)+2O2(g)=CO2(g)+2H20(l) ��H1=akJ/mol�� �����㷴ӦCH4(g)+4NO(g)=CO2(g)+2H20(l)+2N2(g)���ʱ� ��H2����Ҫ��ѯij��Ӧ���ʱ��H3������Ӧ�и����ʵĻ�ѧ������֮��Ϊ���������ʱ����H3=bkJ/mol���÷�Ӧ���Ȼ�ѧ����ʽ��__________________________�ݴ˼������H2= kJ/mol ���ú�a��b��ʽ�ӱ�ʾ����
��3��������������ȥ�����еĵ�������������������в���������Ȼ�������ͨ��ʯ�����У�ʹ֮ת��Ϊ����ơ���֪ij����������NO��NO2��ɣ���n(NO)��n(NO2)=1��3.д���������շ���ȥ��������Ļ�ѧ����ʽ______________________________________���б���µ�O233.6ml�������Ͽ��Դ����õ�������______ml����״���£���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com