
��12�֣��屻��Ϊ������Ԫ�ء�����֪Br2�ķе�Ϊ59�棬����ˮ���ж��Ժ�ǿ��ʴ�ԡ�ʵ����ģ��Ӻ�ˮ����ȡ�����Ҫ����Ϊ��
����1������ˮ����Ũ����ȥ���Σ�
����2������ȥ���κ��ĸҺ�ữ��ͨ��������������ʹBr��ת��ΪBr2��
����3������2���õ�ˮ��Һ��ͨ���ȿ�����ˮ���������嵥�ʴ���ʢ�ж�������ˮ��Һ�������У�
����4�������������ͨ��������������ʹBr��ת��ΪBr2��
����5�������Ȼ�̼��ȡ�嵥�ʣ�����Һ������ô��塣
��1������3�еķ�Ӧ�����ӷ���ʽ �� ��
��2������2���Ѿ��Ƶ����壬��Ҫ���в���3�Ͳ���4��ԭ���� �� ��
��3������5����ȡ�ͷ�Һ����Ҫ����Ҫ��������Ϊ �� ��
��4��������ͼ��ʾ��ʵ��װ�ÿɾ��ƴ��塣
�ٷ�Ӧ��������Ҫ��A�������ȣ����ȵķ����� �� ��
ͼ����ȴˮӦ��B�� �� �ڽ��루�a����b������
��C�мӱ���Ŀ���� �� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣��� �屻��Ϊ����Ԫ�أ�����±��ͨ���������Ƶ��壺Cl2 + 2NaBr = 2NaCl + Br2���÷�Ӧ��������Ԫ��Ϊ ��дԪ�ط��ţ�����������Ӧ�Ƶ�16g Br2 ����ת�Ƶĵ�����Ŀ�� ����
�� �밴Ҫ����д��ѧ����ʽ�����ӷ���ʽ
��1��С�����û�ѧʵ��֤���˾����ڿ����е�Ư���ѱ��ʣ����û�ѧ����ʽ��ʾƯ�۱��ʵ�ԭ�� ��
��2��FeSO4��Һ��ϡH2SO4�ữ������һ��ʱ������Ի�ɫ��д���仯���̵����ӷ���ʽ
��
Ȼ�������еμ�KI-������Һ����ɫ��д���仯���̵����ӷ���ʽ _��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ɽ��������̨���и�һ��ѧ����ĩģ�⿼�Ի�ѧ�Ծ� ���ͣ������
��8�֣��� �屻��Ϊ����Ԫ�أ�����±��ͨ���������Ƶ��壺Cl2 + 2NaBr =" 2NaCl" + Br2���÷�Ӧ��������Ԫ��Ϊ ��дԪ�ط��ţ�����������Ӧ�Ƶ�16g Br2 ����ת�Ƶĵ�����Ŀ�� ����
�� �밴Ҫ����д��ѧ����ʽ�����ӷ���ʽ
��1��С�����û�ѧʵ��֤���˾����ڿ����е�Ư���ѱ��ʣ����û�ѧ����ʽ��ʾƯ�۱��ʵ�ԭ�� ��
��2��FeSO4��Һ��ϡH2SO4�ữ������һ��ʱ������Ի�ɫ��д���仯���̵����ӷ���ʽ
��
Ȼ�������еμ�KI-������Һ����ɫ��д���仯���̵����ӷ���ʽ _��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ������ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
�屻��Ϊ������Ԫ�ء�����֪Br2�ķе�Ϊ59�棬����ˮ���ж��Ժ�ǿ��ʴ�ԡ�ʵ����ģ��Ӻ�ˮ����ȡ�����Ҫ����Ϊ��
����1������ˮ����Ũ����ȥ����
����2������ȥ���κ��ĸҺ�ữ��ͨ��������������ʹBr��ת��ΪBr2��
����3������2����ˮ��Һ��ͨ���ȿ�����ˮ���������嵥�ʴ���ʢ�ж�������ˮ��Һ��������
����4�������������ͨ��������������ʹBr��ת��ΪBr2
����5�������Ȼ�̼��ȡ�嵥�ʣ�����Һ������ô��塣
��1������3�еķ�Ӧ�����ӷ���ʽ ��
��2������2���Ѿ��Ƶ����壬��Ҫ���в���3�Ͳ���4��ԭ���� ��Ԫ�ء�
��3������5����ȡ�ͷ�Һ����Ҫ����Ҫ��������Ϊ ��
��4��������ͼʵ��װ�þ��ƴ��塣
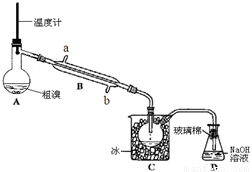
��ͼ����ȴˮӦ��B�� �ڽ���(�a����b��) ��
��C�мӱ���Ŀ���ǽ��£�������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com