
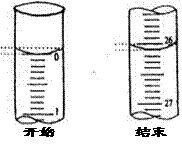
| A����ʽ�ζ���δ�ñ�������Һ��ϴ��ֱ��ע���������Һ |
| B���ζ�ǰʢ������������Һ����ƿ������ˮϴ ����û�и��� |
| C����ʽ�ζ����ڵζ�ǰ�����ݣ��ζ���������ʧ |
| D����ȡ�������ʱ���ζ�����ʱ���Ӷ��� |
|
| �ζ����� | ��������������Һ�����/mL | 0.1000mol/L����������mL�� | ||
| �ζ�ǰ�̶� | �ζ���̶� | ��Һ��/mL | ||
| ��һ�� | 25.00 | 0.00 | 26.11 | 26.11 |
| �ڶ��� | 25.00 | 1.56 | 30.30 | 28.74 |
| ������ | 25.00 | 0.22 | 26.31 | 26.09 |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
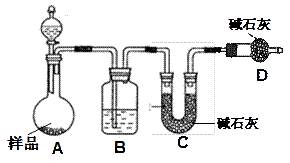
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
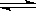 H����ClO����Ϊ��̽��HClO��Ư���ԣ���ͬѧ��������µ�ʵ�顣
H����ClO����Ϊ��̽��HClO��Ư���ԣ���ͬѧ��������µ�ʵ�顣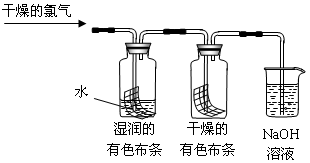
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ü��ȵķ��������Ȼ��ƺ��Ȼ�淋Ĺ������� |
| B����ˮ������ȩ������������ |
| C���ú˴Ź���������δ֪��C2H6O�ķ��ӽṹ |
| D����10mL��Ͳ��ȡ5.00mL1.00 mol��L-1��������0.100mol��L-1���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ζ�ǰ��ƿ������ˮ��ϴ�� |
| B���ζ�ǰ��ƿ�м������������ּ�����ˮ |
| C���ζ�ǰ����������ȷ���ζ����Ӷ��� |
| D���ζ�ǰ����������δ�ų������еζ����ζ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
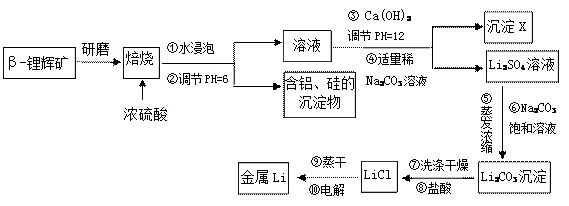
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Һʱ����Һ©�����²�Һ����¿ڷų����ϲ�Һ����Ͽڵ��� |
| B����100mL ��Ͳ��ȡ5.20mL ���� |
| C����������ƽ��ȡ25.20g NaCl���� |
| D����100mL ����ƿ����125mL 0.1mol��L��1���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����SO2ͨ��BaCl2��Һ�в�����ɫ�������ٵμ�ϡ�����������ʧ |
| B���ñ�����������Һ�ζ�һ���������ȵ����ᣬ�յ�ʱ��Һ�ɳ�ɫ��ɻ�ɫ |
| C��ȡ�����ӵ�ʳ�μ��������Һ�У���ֽ������Һ����ɫ |
| D������FeSO4��Һʱ����������ϡ������������ˮ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com