
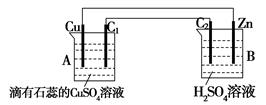


 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
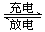 NiOOH + MH������������ȷ���ǣ� ��
NiOOH + MH������������ȷ���ǣ� ��| A���ŵ�ʱ����������Һ�ļ�����ǿ |
| B���ŵ�ʱ������ӦΪ��M + H2O + e���� MH + OH�� |
| C�����ʱ������ӦΪ��NiOOH + H2O + e���� Ni(OH)2 + OH�� |
| D���ŵ�ʱÿת��1mol���ӣ�������1mol NiOOH������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
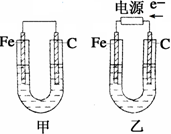
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������ڵ���������ȡ������������������ |
| B����ⷨ������ͭ���ô�ͭ������ |
| C������������ʴ��������Ӧ��O2 +2H2O+4e---=4OH�� |
| D���ڶƼ��ϵ��п����п������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
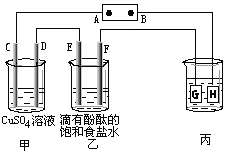
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������¼ܻ�����������һ�����ϲ� | B�������г���Ȧ����һ������� |
| C������բ����ֱ����Դ�������� | D������դ������Ϳ��һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Fe -2e-= Fe2+ | B��Fe -3e- = Fe3+ |
| C��O2 +2H2O +4e- = 4OH- ���� | D��2H+ + 2e- = H2 �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��2Fe-4e ��2Fe2+ |
| B��2Fe2++4e ��2Fe |
| C��2H2O+O2+4e ��4OH�� |
| D��Fe3++e ��Fe2+ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com