
(8��)������ͼ��ʾ(�г�����ʡ��)��װ�ý���ʵ�飬��Һ��A��μ��뵽����B�У��ش��������⣺
����ͼ��Dװ����ʵ���е�������__________________________________________��
����AΪŨ���ᣬBΪKMnO4��CΪ����KI��Һ������E��C�е�����Ϊ____________________����Ӧ�Ļ�ѧ����ʽ��______________________________________��
����AΪŨ����(70%)��BΪNa2SO3��CΪ����KMnO4��Һ������E��C�е�����Ϊ________________________________________________________________________��
��Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
����AΪ30%H2O2��Һ��BΪMnO2��CΪH2S������Һ������E��C�е�����Ϊ________________________________________________________________________��
��Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
����AΪϡ���ᣬBΪ����ʯ��CΪNa2SiO3��Һ������E��C�е�����Ϊ________________����Ӧ�Ļ�ѧ����ʽ��__________________________________��
����ͼ��ʾװ���ж�����;�������(������֮��)��AΪ________��BΪ________��C��ʢ________������E��C�е�����Ϊ________________����Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
�ٷ�ֹ��Һ����'����Һ�ȱ�������ɫ'2KI��Cl2===2KCl��I2��5Cl2��I2��6H2O===2HIO3��10HCl'�۸��������Һ���Ϻ�ɫ��ȥ'5SO2��2H2O��2KMnO4===K2SO4��2MnSO4��2H2SO4'����Һ�в�������ɫ����O2��2H2S===
2S����2H2O'�ݲ�����ɫ��״����'CO2��H2O��Na2SiO3===Na2CO3��H2SiO3��'��Ũ��ˮ'��ʯ��'��������Һ����������Һ'�Թܱ��ϳ���������CH2OH(CHOH)4CHO��
2Ag(NH3)2OHCH2OH(CHOH)4COOH��2 Ag����4NH3����H2O
����:ͬһװ�ã�������;������ѧ���������֪ʶ������������ѧ����Ұ��


 �����ܾ�ϵ�д�
�����ܾ�ϵ�д� ���ƿ�����ϵ�д�
���ƿ�����ϵ�д� ���¿쳵����������ϵ�д�
���¿쳵����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
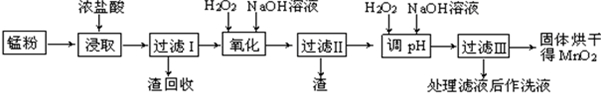
| �� �� | ��ʼ���� | ������ȫ |
| Fe��OH��3 | 2.7 | 3.7 |
| Fe��OH��2 | 7.6 | 9.6 |
| Mn��OH��2 | 8.3 | 9.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��߿���ѧһ�ָ�ϰ����ѧʵ�������ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
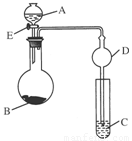
(8��)������ͼ��ʾ(�г�����ʡ��)��װ�ý���ʵ�飬��Һ��A��μ��뵽����B�У��ش��������⣺
����ͼ��Dװ����ʵ���е�������__________________________________________��
����AΪŨ���ᣬBΪKMnO4��CΪ����KI��Һ������E��C�е�����Ϊ____________________����Ӧ�Ļ�ѧ����ʽ��______________________________________��
����AΪŨ����(70%)��BΪNa2SO3��CΪ����KMnO4��Һ������E��C�е�����Ϊ________________________________________________________________________��
��Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
����AΪ30%H2O2��Һ��BΪMnO2��CΪH2S������Һ������E��C�е�����Ϊ________________________________________________________________________��
��Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
����AΪϡ���ᣬBΪ����ʯ��CΪNa2SiO3��Һ������E��C�е�����Ϊ________________����Ӧ�Ļ�ѧ����ʽ��__________________________________��
����ͼ��ʾװ���ж�����;�������(������֮��)��AΪ________��BΪ________��C��ʢ________������E��C�е�����Ϊ________________����Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��߿���ѧһ�ָ�ϰ����ѧʵ�������ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(8��)������ͼ��ʾ(�г�����ʡ��)��װ�ý���ʵ�飬��Һ��A��μ��뵽����B�У��ش��������⣺
����ͼ��Dװ����ʵ���е�������__________________________________________��
����AΪŨ���ᣬBΪKMnO4��CΪ����KI��Һ������E��C�е�����Ϊ____________________����Ӧ�Ļ�ѧ����ʽ��______________________________________��
����AΪŨ����(70%)��BΪNa2SO3��CΪ����KMnO4��Һ������E��C�е�����Ϊ________________________________________________________________________��
��Ӧ�Ļ�ѧ����ʽ��_______________________________________________��
����AΪ30%H2O2��Һ��BΪMnO2��CΪH2S������Һ������E��C�е�����Ϊ________________________________________________________________________��
��Ӧ�Ļ�ѧ����ʽ��__________________________________________________��
����AΪϡ���ᣬBΪ����ʯ��CΪNa2SiO3��Һ������E��C�е�����Ϊ________________����Ӧ�Ļ�ѧ����ʽ��__________________________________��
����ͼ��ʾװ���ж�����;�������(������֮��)��AΪ________��BΪ________��C��ʢ________������E��C�е�����Ϊ________________����Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
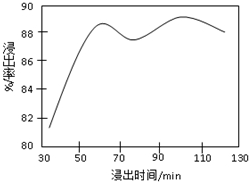
(13��)I�������������������Ϊһ�����ҹ�ҵ����ˮƽ��һ�ֱ�־���±�Ϊ�Ӵ���������ʱ���¶Ⱥ�ѹǿ��SO2ת���ʵ�Ӱ�죺

(1)���Ṥҵ�ڹ�����ռ����Ҫ�ĵ�λ����д������������Ҫ��;�� �� ��
(2)��ҵ�д�����SO2���ó�ѹ�����ø�ѹ��������
II��ʵ�����У�������ͼ��ʾװ�ü�����ҩƷ(ͼ�в��ּг���������ȥ)̽����ҵ������Ӵ����еķ�Ӧ�����ռ��õ�SO3����֪SO3�۵�Ϊ16��8�棬�е�44��8�棬��SO3��ˮ���ҷ�Ӧ��

(1)д��Aװ������ȡ�����Ļ�ѧ����ʽ�� ��
(2)Bװ�õ����ó��˽������ֻ���⣬���У�
�� ���� ��
��3������Ӳ�ʲ�����ʱ�������������¶ȣ���SO2����O2����SO3�Ļ�ѧ��Ӧ�к�Ӱ��
(4)��ʵ��װ��������һװ�ã��ס��ҡ�������ͬѧ�ֱ������a��b��c����װ������Ϊ���������� �������� ��

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com