
| 448��10-3L |
| 22.4L/mol |
| 1.52g |
| 76g/mol |
| 3.6g |
| 18g/mol |
| 6.6g |
| 44g/mol |
| 6.6g |
| 44g/mol |
| 3.6g |
| 18g/mol |
| ˮԡ���� |
| ˮԡ���� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
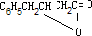
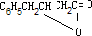
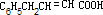
| Ũ���� |
| �� |
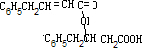 +H2O��C6H5CH2CH��OH��CH2COOH+
+H2O��C6H5CH2CH��OH��CH2COOH+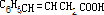
| Ũ���� |
| �� |
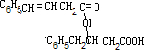 +H2O
+H2O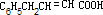
| Ũ���� |
| �� |
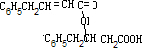 +H2O��C6H5CH2CH��OH��CH2COOH+
+H2O��C6H5CH2CH��OH��CH2COOH+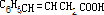
| Ũ���� |
| �� |
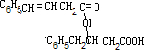 +H2O
+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
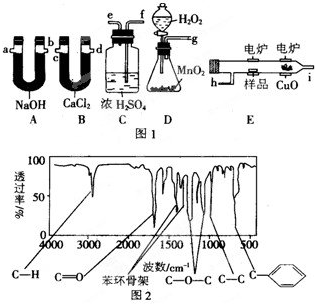
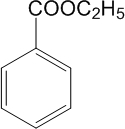

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
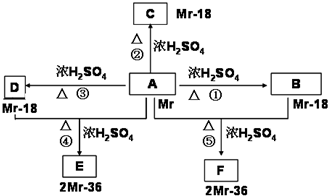
��֪�л���A�����к��б�����ֻ��һ���������л���A����Է�������M������200��������Ԫ�ص���������Ϊ26.7%����ȫȼ��ֻ����ˮ�Ͷ�����̼����֮�йص��л���ת����ϵ���£���ע�⣺���ַ�Ӧ����ʡ�ԣ�
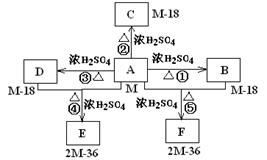
��1���л���A�к��й����ŵ����Ʒֱ�Ϊ________________��A�ķ���ʽΪ____________��
��2���л���õĽṹ��ʽΪ�ߣߣߣߣߣߣߣ�
��3���л���A��F�л�Ϊͬ���칹�����____________��______________����д��ţ�
��4���л���A��F�в���ʹ������Ȼ�̼��Һ��ɫ����_________________����д��ţ�
��5��16.2g�л���BCD��ɵĻ������ȫȼ�գ��õ���CO2ͨ��3mol/LNaOH��Һ�еõ��IJ���Ϊ________________��
��6��д��A+D����E�Ļ�ѧ����ʽ_____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ����ʦԭ�����ȫ ���ͣ��ƶ���
��֪�л���A�����к��б�����ֻ��һ���������л���A����Է�������M������200��������Ԫ�ص���������Ϊ26.7%����ȫȼ��ֻ����ˮ�Ͷ�����̼����֮�йص��л���ת����ϵ���£���ע�⣺���ַ�Ӧ����ʡ�ԣ�
��1���л���A�к��й����ŵ����Ʒֱ�Ϊ________________��A�ķ���ʽΪ____________��
��2���л���õĽṹ��ʽΪ�ߣߣߣߣߣߣߣ�
��3���л���A��F�л�Ϊͬ���칹�����____________��______________����д��ţ�
��4���л���A��F�в���ʹ������Ȼ�̼��Һ��ɫ����_________________����д��ţ�
��5��16.2g�л���BCD��ɵĻ������ȫȼ�գ��õ���CO2ͨ��3mol/LNaOH��Һ�еõ��IJ���Ϊ________________��
��6��д��A+D����E�Ļ�ѧ����ʽ_____________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com