
�蹸���ɷ�ֹ��ҵ��ˮ��������������Ṹ���������з�Ӧ·�߿ɵõ�E��R�����蹸��(���ַ�Ӧ������ȥ)��
(1)�蹸��E���Ʊ�
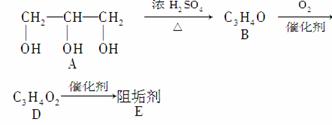
��A����������Ҫ��Ӫ������________ˮ���Ƶá�(����ࡱ������֬�������ʡ�)
��B�����Ƶ�Cu(OH)2����Һ��Ӧ����D���仯ѧ����ʽΪ
________________________________________________________________________
________________________________________________________________________��
��D���Ӿ۷�Ӧ����E��E�Ľṹ��ʽΪ
________________________________________________________________________
________________________________________________________________________��
(2)�蹸��R���Ʊ�
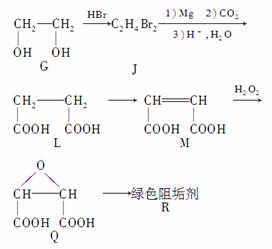
��G�D��JΪȡ����Ӧ��J�Ľṹ��ʽΪ
________________________________________________________________________��
��Jת��ΪL�Ĺ����У�L���������ӵ�̼ԭ����Դ��__________��
����L�Ʊ�M�ķ�Ӧ��������Ϊ��HOOCCH2CH2COOH��Br2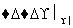
HOOCCH2CHBrCOOH��HBr��______________________
________________________________________________________________________��
________________________________________________________________________
_______________________________________(�û�ѧ����ʽ��ʾ)��
��1 mol Q��ͬ���칹��T(̼����֧��)������NaHCO3��Һ���ò���2 mol CO2��T�Ľṹ��ʽΪ__________________________________(ֻдһ��)��
��(1)����֬����CH2===CHCHO��2Cu(OH)2CH2===CHCOOH��Cu2O����2H2O
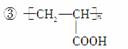
(2)��CH2BrCH2Br����CO2
��HOOCCH2CHBrCOOH��3NaOH
NaOOCCH===CHCOONa��NaBr��3H2O
NaOOCCH===CHCOONa��H2SO4�D��
HOOCCH===CHCOOH��Na2SO4
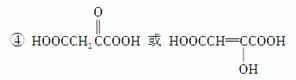
������(1)��AΪ���ͣ�������֬(��֬����ĸ�����)ˮ���Ƶá�����A��B�ķ���ʽ�仯��B������Cu(OH)2����Һ��Ӧ����Ϣ����֪��B(C3H4O)�Ľṹ��ʽΪCH2===CHCHO�����ɺϳ�·�߿���֪DΪ
CH2===CHCOOH��EΪ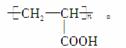
(2)��G�D��JΪ�����������ȡ����Ӧ����JΪCH2BrCH2Br��
�ڸ���Ԫ���غ㶨�ɣ���֪L���������ӵ�̼ԭ����Դ��CO2��
����L�Ʊ�M�Ĺ���Ϊ��ȡ������±��ԭ�ӣ�����ȥ����̼̼˫��������ữ����ԭ���Ȼ���
��T��Q��Ϊͬ���칹�壬��T�ķ���ʽΪC4H4O5��1 mol T������NaOH��Һ��������2 mol CO2������֪T�к���2���Ȼ�������ṹ��ʽ����Ϊ
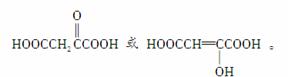


 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����4���л����У������ںϳɽṹ��ʽΪ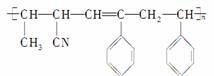 �ĸ߷��Ӳ��ϵ���(����)
�ĸ߷��Ӳ��ϵ���(����)
��CCH2CH3CN����CHCH2
��CCH����CH3��CH==CH��CN
A���٢ۢ� B���٢ڢ�
C���٢ڢ� D���ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪C(s)��H2O(g)��CO(g)��H2(g) ��H��+130kJ��mol��1
2C(s)��O2(g)��2CO(g) ��H����220kJ��mol��1
H��H��O��O��O��H���ļ��ֱܷ�Ϊ436 kJ��mol��1��a kJ��mol��1��462kJ��mol��1,
��aΪ
A��496 B��118 C��350 D��130
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij���������������¿��Է���ˮ�ⷴӦ������������A��B����A��B����Է���������ȣ������ʿ�����(����)
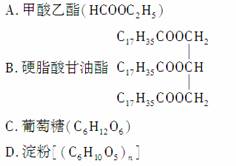
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ڲ�ͬ�����¿��Ա������ɲ�ͬ���ʡ���������ش����⣺
��֪RCOOH��CH2===CH2�� O2
O2 RCOOCH===CH2��H2O
RCOOCH===CH2��H2O
(1)�������ھƻ�ø�����������л���A��A��B��C��D��E���ת����ϵ��ͼ��ʾ��
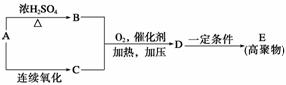
��B��ʯ�ͻ�ѧ��ҵ����Ҫ�Ļ���ԭ�ϣ�д��A�D��B�Ļ�ѧ����ʽ��
________________________________________________________________________
________________________________________________________________________��
��D�Ľṹ��ʽΪ
________________________________________________________________________��
(2)��������һ�������»��ɱ�����ΪX��Y(Y��A����Է���������ͬ)��X�ɴ�������Y��Ҳ����H2��Ӧ����Z��X��Y�Ľṹ����һ����ͬ�Ĺ�������____________������˹��������õ��Լ���________________________________________��
(3)F�����弡��ϸ���е���������ȱ�������½������������IJ��F��G��H���ת����ϵ��F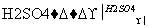 G
G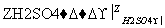 H��H��(1)�е�D��Ϊͬ���칹�塣
H��H��(1)�е�D��Ϊͬ���칹�塣
��G�����Է����ķ�Ӧ��________(�����)
��.�ӳɷ�Ӧ ��.ˮ�ⷴӦ
��.������Ӧ ��.��ȥ��Ӧ
��.��ԭ��Ӧ
�ڱ����漰�������л����У���F�����Ժ��������Ȼ��(������һ��)����ȫȼ������CO2��H2O�����ʵ����������(д���ṹ��ʽ)____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
X��Y��ΪԪ�����ڱ���ǰ20��Ԫ��,������ӵĵ��Ӳ�ṹ��ͬ,����˵����ȷ����(��)
A.X2-�Ļ�ԭ��һ ������Y-
������Y-
B.��mXa+��nYb-��,m+a=n-b
C.X��Yһ������ͬ����Ԫ��
D.��X��ԭ�Ӱ뾶����Y,����̬�⻯����ȶ���һ����X����Y
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����ԭ�ӻ�ԭ���Ų����ڹ����ŵ��ǣ� ��
A��NO3- B����NO2 C���� OH D����CHO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ɱ���ϩ��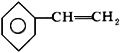 �����ǻ�����������
�����ǻ�����������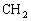 �C
�C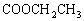 ����ɵ�
����ɵ�
OH
������У���̼Ԫ�ص���������Ϊ70%����ô��Ԫ�ص���������ԼΪ
A��4.6% B��7.7% C��15.6% D��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ȷ���ǣ� ��
A�����ۡ���ά�ء���֬�����ڸ߷��ӻ�����
B���ܷ���������Ӧ���һ����������
C����Ȼ����ˮ������ղ����Ϊ������
D����֬ˮ��õ��Ĵ��DZ�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com