
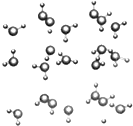
 ��Ϣ����20�桢1������ѹ�£�ˮ���Խ�ɱ�����Ϊ���ȱ�������ͼ����
��Ϣ����20�桢1������ѹ�£�ˮ���Խ�ɱ�����Ϊ���ȱ�������ͼ�������� ��1��ˮ������Hԭ�ӵ�s�����Oԭ�ӵ�p���ͷ��ͷ�ص���
��2���ȱ����ڷ��Ӿ��壬�ȱ��з��Ӽ���ڷ��Ӽ��������������
��3�����ݼ۲���ӶԻ�������ȷ�����ӵĿռ乹�ͣ�1molH2O���γ�2mol�����
��4��ԭ�Ӹ�����ȡ��۵�������ȵ���Ϊ�ȵ����壮
��� �⣺��1��H2O��������ԭ�ӵ�s�������ԭ�ӵ�sp3�ӻ������ͷ��ͷ�ص������Ժ��еĹ��ۼ��÷��ű�ʾΪ��s-sp3���sp3-s��
�ʴ�Ϊ����s-sp3���sp3-s��
��2���ȱ����ڷ��Ӿ��壬���Ӽ���ڷ��Ӽ��������������
A�����ʯ����ԭ�Ӿ��壬���ʯ��ֻ�����ۼ����ۻ�ʱֻ�ƻ����ۼ�����A����
B���ɱ����ڷ��Ӿ��壬���ɱ���ֻ�����Ӽ�����������������ۻ�ʱֻ�ƻ����Ӽ�����������B����
C��ʳ���������Ӿ��壬ʳ����ֻ�����Ӽ����ۻ�ʱֻ�ƻ����Ӽ�����C����
D����̬�����ڷ��Ӿ��壬�����д��ڷ��Ӽ�������������������ۻ�ʱ�ƻ����Ӽ����������������D��ȷ��
��ѡD��
��3��ˮ������������ԭ�Ӻ���3�����۵�����1���µ��Ӷԣ�������ռ乹��Ϊ�����Σ���1molH2O�ı��������γɡ������2mol��
�ʴ�Ϊ�������Σ�2��
��4��ԭ�Ӹ�����ȡ��۵�������ȵ���Ϊ�ȵ����壬������Ԫ��ԭ���γɵ���H3O+��Ϊ�ȵ�����ķ��ӻ�������NH3���ʴ�Ϊ��NH3��
���� ���⿼�������ʽṹ�����ʣ��漰���Ӽ���������������ȵ������֪ʶ�㣬�������ʵľ�������ȷ�����ڵĻ�ѧ����������ɣ�ע����������ڻ�ѧ����Ϊ�״��㣮


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʯ��ׯ������ѧ�߶��Ͻο�һ��ѧ���������棩 ���ͣ�ѡ����
��100 mL pH = l��H2SO4��Һ�У��μ�0.01 mol•L��1 NaOH��Һ�������û��Һ��pHΪ2ʱ������NaOH��Һ������ǣ� ��
A��10 mL B��90 mL C��100 mL D��450 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�켪��ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
ij�л�����ҩ���������м��壬��ṹ��ʽ����ͼ�������й�������ȷ����

A�����л�������ˮ�����ӳɷ�Ӧ
B�����л�����Ũ�����ϼ��ȿɷ�����ȥ��Ӧ
C��1mol���л���������NaOH��Һ��Ӧ�������3molNaOH
D�����л��ᆳ���������ܷ���������Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
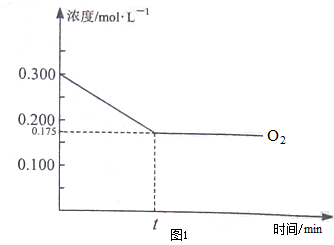

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na+�Ľṹʾ��ͼΪ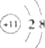 | |
| B�� | ����Ļ�ѧʽΪNa2CO3 | |
| C�� | �����������£�����ˮ��Ļ�ѧ����ʽΪC12H20O11+H2O��2C6H12O6�������ǣ� | |
| D�� | �����ᣨHClO4������Ԫ�صĻ��ϼ�Ϊ+7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ĸ������Կ����е��Ĺ̶� | |
| B�� | ��NO2������ȴ����ɫ���dz | |
| C�� | ͨ��ú��Һ������ȡ�������ױ��Ȼ���ԭ�� | |
| D�� | ��ҵ��Һ̬�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | һ��������6.4g SO2������������Ӧ����SO3��ת�Ƶ�����Ϊ0.2 NA | |
| B�� | 1 mol AlCl3������״̬ʱ��������Ϊ0.4NA | |
| C�� | ����£�11.2 L������ȫ����1 Lˮ�У�������Һ��Cl-��ClO-����������֮��ΪNA | |
| D�� | 6.4 g��S2��S4��S8��ɵĻ���ﺬ��ԭ����Ϊ0.2 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ˮ�������ӵĵ���ʽ��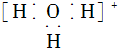 | B�� | �����ӵĽṹʾ��ͼ�� | ||
| C�� | ������Ϊ28�ĸ�ԭ�ӣ�2028Ca | D�� | �۱�ϩ�Ľṹ��ʽ��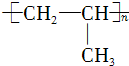 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com