
��9�֣���25��ʱ������0.1mol��L-1�İ�ˮ����ش��������⣺
��1������ˮ�м�����������粒��壬��ʱ��Һ���� �����������С�����䡱����
��2������ˮ�м���pH=1�����ᣬ�Ұ�ˮ������������Ϊ1��1����ʱ��Һ��pH 7������ڡ�����С�ڡ����ڡ����������ӷ���ʽ��ʾ��ԭ��
����ʱ��Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
��3������ˮ�м���0.05mol��L-1ϡ��������Һ���ó����ԣ����ð�ˮ�����V1��ϡ��������V2�Ĺ�ϵΪV1 V2������ڡ�����С�ڡ����ڡ�����д����Һ�и�����Ũ��֮������ĵ���غ����ʽ ��
(1)����1�֣�
��2��С�ڣ�1�֣� NH4+ +H2ONH3��H2O+H+��2�֣�
c(NH4+)��c(SO42- )��c(H+)��c(OH-)(2�֣�
��3�����ڣ�1�֣� c(NH4+)+c(H+)=2c(SO42- )+c(OH-)��2�֣�
����:��1����ˮ�д��ڵ���ƽ�⣺NH3��H2ONH4+ +OH-������ˮ�м�����������粒��壬�������c(NH4+)�����Ƶ��룬��ƽ���c(NH4+)�Ա�ԭƽ�����Һ��
������
��2��pH=1�����ᣬ��Ũ����0.05mol��L-1��ͨ�������֪�������϶���ǡ�÷�Ӧ����������泥������ˮ�������ԡ�����ʽΪNH4+ +H2ONH3��H2O+H+��
��3���ɣ�2����֪�������Ϻ���Һ�����ԣ�����Ҫ����Һ�����ԣ���ˮ�������������ˮ�������������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| c(NH+) | c(NH3?H2O) |
 NH3?H2O+H+
NH3?H2O+H+ NH3?H2O+H+
NH3?H2O+H+�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��9�֣���25��ʱ������0.1mol��L-1�İ�ˮ����ش��������⣺
��1������ˮ�м�����������粒��壬��ʱ��Һ���� �����������С�����䡱����
��2������ˮ�м���pH=1�����ᣬ�Ұ�ˮ������������Ϊ1��1����ʱ��Һ��pH 7������ڡ�����С�ڡ����ڡ����������ӷ���ʽ��ʾ��ԭ��
����ʱ��Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
��3������ˮ�м���0.05mol��L-1ϡ��������Һ���ó����ԣ����ð�ˮ�����V1��ϡ��������V2�Ĺ�ϵΪV1 V2������ڡ�����С�ڡ����ڡ�����д����Һ�и�����Ũ��֮������ĵ���غ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ʡ�߶���ѧ����ĩ������ѧ�Ծ��������棩 ���ͣ������
��9�֣���25��ʱ������0.1mol��L-1�İ�ˮ����ش��������⣺
��1������ˮ�м�����������粒��壬��ʱ��Һ��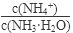 ��
�����������С�����䡱����
��
�����������С�����䡱����
��2������ˮ�м���pH=1�����ᣬ�Ұ�ˮ������������Ϊ1��1����ʱ��Һ��pH 7������ڡ�����С�ڡ����ڡ����������ӷ���ʽ��ʾ��ԭ��
����ʱ��Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
��3������ˮ�м���0.05mol��L-1ϡ��������Һ���ó����ԣ����ð�ˮ�����V1��ϡ��������V2�Ĺ�ϵΪV1 V2������ڡ�����С�ڡ����ڡ�����д����Һ�и�����Ũ��֮������ĵ���غ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ�߶���һ���¿���ѧ�Ծ� ���ͣ������
��9�֣���25��ʱ������0.1mol��L-1�İ�ˮ����ش��������⣺
��1������ˮ�м�����������粒��壬��ʱ��Һ�� ��
�����������С�����䡱����
��
�����������С�����䡱����
��2������ˮ�м���pH=1�����ᣬ�Ұ�ˮ������������Ϊ1��1����ʱ��Һ��pH 7������ڡ�����С�ڡ����ڡ����������ӷ���ʽ��ʾ��ԭ��
����ʱ��Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
��3������ˮ�м���0.05mol��L-1ϡ��������Һ���ó����ԣ����ð�ˮ�����V1��ϡ��������V2�Ĺ�ϵΪV1 V2������ڡ�����С�ڡ����ڡ�����д����Һ�и�����Ũ��֮������ĵ���غ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��������̨���и߶���ѧ����ĩ��ǰģ�⻯ѧ�Ծ� ���ͣ������
��9�֣���25��ʱ������0.1mol��L-1�İ�ˮ����ش��������⣺
��1������ˮ�м�����������粒��壬��ʱ��Һ��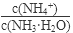 ��
�����������С�����䡱����
��
�����������С�����䡱����
��2������ˮ�м���pH=1�����ᣬ�Ұ�ˮ������������Ϊ1��1����ʱ��Һ��pH 7������ڡ�����С�ڡ����ڡ����������ӷ���ʽ��ʾ��ԭ��
����ʱ��Һ�и�����Ũ���ɴ�С��˳��Ϊ ��
��3������ˮ�м���0.05mol��L-1ϡ��������Һ���ó����ԣ����ð�ˮ�����V1��ϡ��������V2�Ĺ�ϵΪV1 V2������ڡ�����С�ڡ����ڡ�����д����Һ�и�����Ũ��֮������ĵ���غ����ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com