
��ͼ���漰�����ʾ�Ϊ��ѧ��ѧ�еij������ʣ����У���C��D��E��F��Ϊ���ʣ�����Ϊ�������C��D��E �ڳ�����Ϊ���壬��������Ϊ�����Һ�壻��A��I ���ֻ��������ɫ��Ӧ�ֱ�Ϊ��ɫ�ͻ�ɫ���ܷ�Ӧ�١����е�һЩ�������Ѿ���ȥ����Щ��Ӧ������δ�г������Ǵ�������ת����ϵ��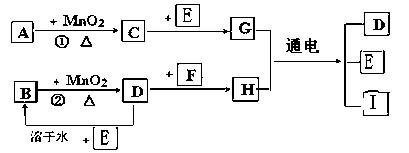
�� д���й����ʵĻ�ѧʽ��
A ��D ��F ��I ��
�� ָ��MnO2����ط�Ӧ�е����ã���Ӧ������ ������Ӧ������ ����
�� д��B��MnO2���Ȼ��D�Ļ�ѧ����ʽ____________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ���漰�����ʾ�Ϊ��ѧ��ѧ�еij������ʣ����Ǵ�������ת����ϵ��
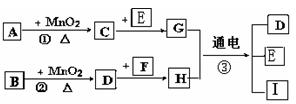
���У�1��C��D��E��F��Ϊ���ʣ�����Ϊ�����2��C��D��E �ڳ�����Ϊ���壬GΪҺ��
��3��A��I ���ֻ��������ɫ��Ӧ��Ϊ��ɫ
��4����Ӧ�٢ڵ�һЩ�������Ѿ���ȥ����Щ��Ӧ������δ�г�
��1��д���й����ʵĻ�ѧʽ��
A �� I ��
��2��ָ��MnO2����ط�Ӧ�е����ã���Ӧ �� ���� ������Ӧ �� ���� ����
��3��д����Ӧ�ڻ�ѧ����ʽ�����������ת�Ƶķ������Ŀ ______ ��
��4��д����Ӧ�۵����ӷ���ʽ______________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�ٺ��и�����ѧ�ڵ�һ���¿���ѧ����� ���ͣ������
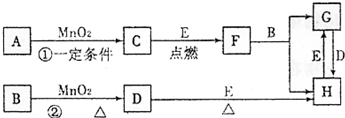
��12�֣���ͼ���漰�����ʾ�Ϊ��ѧ��ѧ�еij������ʣ�����CΪO2��DΪC12��EΪFe���ʣ�����Ϊ��������Ǵ�������ת����ϵ����Ӧ�����ɵ�ˮ����Ҫ���������ȥ��

��1��д���й����ʵ����ƻ�ѧʽ��F ��H ��
����Ӧ�����ڼ��������½��У���A�� ������Ӧ�����ڳ��������½��У���A�� ��
��2��д��B��MnO2���Ȼ��D�����ӷ���ʽ ��
��3��B��ϡ��Һ��AgNO3��Һ��Ͽ��γɳ���AgX���˳�����Ksp(AgX) =1.8��10��10�����������Bϡ��Һ��AgNO3��Һ��ϣ���B��Ũ��Ϊ2��10��4mo1/L�������ɳ�������AgNO3��Һ����СŨ��Ϊ________������AgX����Һ�еμ�KI��Һ���۲쵽������ �������ܹ�����ת����ԭ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com