
Cl2ŗĶH2O2ŹĒøßÖŠ½×¶Ī×ī³£¼ūµÄĮ½ÖÖŃõ»Æ¼Į£¬¾²éŌÄ׏ĮĻCl2Ńõ»ÆÄÜĮ¦ĒæÓŚH2O2£¬Äܽ«H2O2Ńõ»Æ”£ĪŖĮĖŃéÖ¤øĆ½įĀŪ£¬Ń§ÉśÉč¼ĘĮĖČēĻĀĶ¼ĖłŹ¾µÄŹµŃé×°ÖĆ½ųŠŠŹµŃé£Ø¼Š³Ö×°ÖĆĀŌČ„£©”£Ō²µ×ÉÕĘæAÖŠµÄ·“Ó¦·½³ĢŹ½ĪŖ2KMnO4+16HCl£ØÅØ£©=2KCl+2MnCl2+5Cl2”ü+8H2O£¬Ēė»Ų“šĻĀĮŠĪŹĢā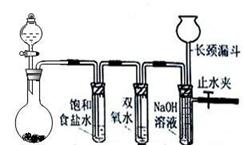
A B C D
£Ø1£©ŹŌ¹ÜBÖŠ±„ŗĶŹ³ŃĪĖ®µÄ×÷ÓĆ £»
£Ø2£©ŹŌ¹ÜCÖŠ¼ÓČė5mL 30% Ė«ŃõĖ®£¬·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½____________________£»
£Ø3£©ŹŌ¹ÜDÖŠ³äĀś10% NaOHČÜŅŗ£¬NaOHČÜŅŗµÄ×÷ÓĆŹĒ £»
£Ø4£©½«ÅØŃĪĖį×¢ČėŌ²µ×ÉÕĘæA£¬µ±×°ÖĆÖŠµÄæÕĘų»ł±¾Åž”ŗó¹Ų±ÕÖ¹Ė®¼Š£¬·“Ó¦Ņ»¶ĪŹ±¼äŗóŹŌ¹ÜDÖŠµÄĻÖĻóĪŖ £¬ŹŌ¹ÜDÖŠµÄĘųĢå¼ģŃé·½·ØĪŖ__________________£»
£Ø5£©ÓŠµÄĶ¬Ń§¶ŌÉĻŹöŹµŃéÖŠŹŌ¹ÜDÖŠĘųĢåĄ“Ō“²śÉśÖŹŅÉ£¬ÄćČĻĪŖæÉÄܵĥ“Ō“ÓŠ £ØÓĆ»Æѧ·½³ĢŹ½±ķŹ¾£©£¬¶ŌÓŚÖŹŅÉæÉŅŌ²ÉÓƶŌ±ČŹµŃ饓½ā¾ö”£
£Ø1£©³żČ„Cl2ÖŠ»ģÓŠµÄHCl£»£Ø2£©Cl2+H2O2=2HCl+O2£»£Ø3£©ĪüŹÕ¶ąÓąµÄCl2£»£Ø4£©ŹŌ¹ÜDÖŠŅŗĆęĻĀ½µ£¬³¤¾±Ā©¶·ÖŠŅŗĆęÉĻÉż£»øĆĘųĢåÄÜŹ¹“ų»šŠĒµÄľĢõø“Č¼£¬Ö¤Ć÷ŹĒŃõĘų£»£Ø5£©2H2O2=2H2O+O2”ü£»Cl2+H2O="HCl+HClO" £»2HClO=2HCl+O2”ü”£
½āĪöŹŌĢā·ÖĪö£ŗ£Ø1£©ĀČ»ÆĒā¼«Ņ×ČÜÓŚĖ®£¬ĀČĘųÄÜČÜÓŚĖ®£¬ĒŅÓėĖ®·“Ó¦£¬Cl2+H2O=H++Cl-+HClO£¬Ź³ŃĪĖ®ČÜŅŗÖŠµÄĀČĄė×ÓŅÖÖĘĀČĘųµÄČܽā£¬½µµĶĀČĘųµÄČܽā¶Č£¬¹Ź±„ŗĶŹ³ŃĪĖ®µÄ×÷ÓĆŹĒ³żČ„Cl2ÖŠ»ģÓŠµÄHCl£»£Ø2£©Cl2Ńõ»ÆÄÜĮ¦ĒæÓŚH2O2£¬Äܽ«H2O2Ńõ»Æ²śÉśO2£¬»Æѧ·½³ĢŹ½Cl2+H2O2=2HCl+O2£Ø3£©ÓŠÉŁĮæµÄCl2ƻӊ·¢Éś·“Ó¦Åųö£¬ÓĆNaOHČÜŅŗĪüŹÕ¶ąÓąµÄCl2£Ø4£©½«ÅØŃĪĖį×¢ČėŌ²µ×ÉÕĘæŗó²śÉś“óĮæµÄĀČĘų£¬ŗóÓėĖ«ŃõĖ®·“Ӧɜ³É“óĮæµÄŃõĘų£¬D֊װÖĆÖŠæÕĘųÅŽńŗóÖ¹Ė®¼Š¹Ų±Õ£¬D×°ÖĆÄŚŃ¹ĒæŌö“óŹ¹ŅŗĆęĻĀ½µ£¬³¤¾±Ā©¶·ÖŠŅŗĆęÉĻÉż£»¼ģŃéŃõĘųµÄ·½·Ø£¬Ź¹“ų»šŠĒµÄľĢõø“Č¼£Ø5£©2H2O2=2H2O+O2”ü£»Cl2+H2O=HCl+HClO£¬2HClO=2HCl+O2”ü”£
æ¼µć£ŗĀČĘųµÄŹµŃéŹŅÖĘ·Ø£»Ńõ»ÆŠŌĒæČõµÄ±Č½Ļ£»ŃõĘųµÄ¼ģŃé·½·Ø



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ŅŃÖŖŅŅ“¼æÉŅŌŗĶĀČ»ÆøĘ·“Ӧɜ³ÉĪ¢ČÜÓŚĖ®µÄCaCl2”¤6C2H5OH”£ÓŠ¹ŲµÄÓŠ»śŹŌ¼ĮµÄ·ŠµćČēĻĀ£ŗCH3COOC2H5ĪŖ77.1”ę£»C2H5OHĪŖ78.3”ę£»C2H5OC2H5£ØŅŅĆŃ£©ĪŖ34.5”ę£»CH3COOHĪŖ118”ę”£ŹµŃéŹŅŗĻ³ÉŅŅĖįŅŅõ„“Ö²śĘ·µÄ²½ÖčČēĻĀ£ŗŌŚÕōĮóÉÕĘæÄŚ½«¹żĮæµÄŅŅ“¼ÓėÉŁĮæÅØĮņĖį»ģŗĻ£¬Č»ŗó¾·ÖŅŗĀ©¶·±ßµĪ¼Ó“×Ėį£¬±ß¼ÓČČÕōĮó”£ÓÉÉĻĆęµÄŹµŃéæɵƵ½ŗ¬ÓŠŅŅ“¼”¢ŅŅĆŃ”¢“×ĖįŗĶĖ®µÄŅŅĖįŅŅõ„“Ö²śĘ·”£
£Ø1£©·“Ó¦ÖŠ¼ÓČėµÄŅŅ“¼ŹĒ¹żĮæµÄ£¬ĘäÄæµÄŹĒ ”£
£Ø2£©±ßµĪ¼Ó“×Ėį£¬±ß¼ÓČČÕōĮóµÄÄæµÄŹĒ ”£
½«“Ö²śĘ·ŌŁ¾ĻĀĮŠ²½Öč¾«ÖĘ£ŗ
£Ø3£©ĪŖ³żČ„ĘäÖŠµÄ“×Ėį£¬æÉĻņ²śĘ·ÖŠ¼ÓČė £ØĢī×ÖÄø£©”£
A.ĪŽĖ®ŅŅ“¼ B.Ģ¼ĖįÄĘ·ŪÄ© C.ĪŽĖ®“×ĖįÄĘ
£Ø4£©ŌŁĻņĘäÖŠ¼ÓČė±„ŗĶĀČ»ÆøĘČÜŅŗ£¬Õńµ“£¬·ÖĄė£¬ĘäÄæµÄŹĒ ”£
£Ø5£©Č»ŗóŌŁĻņĘäÖŠ¼ÓČėĪŽĖ®ĮņĖįĶ£¬Õńµ“£¬ĘäÄæµÄŹĒ ”£×īŗ󣬽«¾¹żÉĻŹö“¦ĄķŗóµÄŅŗĢå¼ÓČėĮķŅ»øÉŌļµÄÕōĮóĘæÄŚ£¬ŌŁÕōĮó£¬ĘśČ„µĶ·ŠµćĮó·Ö£¬ŹÕ¼Æ·ŠµćŌŚ76”ę~78”ęÖ®¼äµÄĮó·Ö¼“µĆ“æ¾»µÄŅŅĖįŅŅõ„”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø12·Ö£©ĢģČ»Ė®ŹĒČĖĄąŅūÓĆĖ®µÄÖ÷ŅŖĄ“Ō“”£“ÓĢģČ»Ė®»ńµĆæÉŅŌŅūÓƵÄĖ®Ņ»°ćŠė¾¹ż³Į½µŠüø”Īļ”¢É±¾śĻū¶¾µČ²½Öč”£
£Ø1£©³Į½µŠüø”ĪļŅŖŌŚĖ®ÖŠ¼ÓČėŠõÄż¼Į£¬Čē½«ĀĮŃĪ¼ÓČėĖ®ÖŠÄÜ“ļµ½¾»Ė®ÄæµÄ£¬
ŌŅņŹĒ £ØÓĆĄė×Ó·½³ĢŹ½±ķŹ¾£©”£
£Ø2£©ĀČĘųæÉÓĆÓŚ×ŌĄ“Ė®É±¾śĻū¶¾¼Į£¬½įŗĻĄė×Ó·½³ĢŹ½ŗĶĪÄ×ÖĄķÓÉ ”£
£Ø3£©ŠĀŠĶĖ®“¦Ąķ¼ĮøßĢśĖį¼Ų (K2FeO4)¾ßÓŠĒæµÄŃõ»Æ×÷ÓĆŗĶŠõÄż×÷ÓĆ”£¹¤ŅµÉĻæÉĶعżŅŌĻĀĮ÷³ĢÖʱøøßĢśĖį¼Ų£ŗ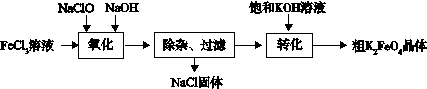
²éŌÄ׏ĮĻ:øßĢśĖįŃĪŌŚÖŠŠŌ»ņĖįŠŌČÜŅŗÖŠ»įÖš½„·Ö½ā£¬ŌŚ¼īŠŌČÜŅŗÖŠĪČ¶Ø”£
Ķź³É”°Ńõ»Æ”±¹ż³ĢÖŠµÄĄė×Ó·½³ĢŹ½
”õFe3+ + ”õClO- +”õ ="”õ" FeO42- + ”õCl- + ”õ
”°×Ŗ»Æ”±¹ż³ĢÖŠŹµĻÖÓÉNa2FeO4ÖʵĆK2FeO4£¬ŹĒĄūÓƶžÕß ŠŌµÄ²»Ķ¬”£
¢Ū½įŗĻ׏ĮĻĶź³É“ÖK2FeO4¾§ĢåµÄĢį“æ£ŗ½«“Ö²śĘ·ÓĆ Čܽā£¬Č»ŗóŌŁ¼ÓČė±„ŗĶKOHČÜŅŗ”¢ĄäČ“½į¾§”¢¹żĀĖ”£
¢ÜøßĢśĖį¼ŲµÄÓ¦ÓĆ»¹ŌŚ²»¶ĻĄ©Õ¹ÖŠ”£ČēæÉÖĘ³ÉøßĢśµē³Ų£¬ µē³Ų·“Ó¦ĪŖ£ŗ
3Zn + 2K2FeO4 + 8H2O  3Zn(OH)2 + 2Fe(OH)3 + 4KOH
3Zn(OH)2 + 2Fe(OH)3 + 4KOH
·ÅµēŹ±£¬Õż¼«·“Ó¦ĪŖ£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
CaCO3¹ć·ŗ“ęŌŚÓŚ×ŌČ»½ē£¬ŹĒŅ»ÖÖÖŲŅŖµÄ»Æ¹¤ŌĮĻ”£“óĄķŹÆÖ÷ŅŖ³É·ÖĪŖCaCO3£¬ĮķĶāÓŠÉŁĮæµÄŗ¬Įņ»ÆŗĻĪļ(ČēFeSµČ)”£ŹµŃéŹŅÓĆ“óĄķŹÆŗĶĻ”ŃĪĖį·“Ó¦ÖʱøCO2ĘųĢ唣ĻĀĮŠ×°ÖĆæÉÓĆÓŚCO2ĘųĢåµÄĢį“æŗĶøÉŌļ”£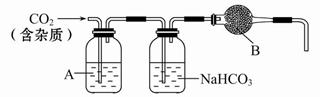
Ķź³ÉĻĀĮŠĢīæÕ£ŗ
(1)ÓĆÅØŃĪĖįÅäÖĘ1”Ć1(Ģå»ż±Č)µÄĻ”ŃĪĖį(Ō¼6 mol”¤L-1)£¬Ó¦Ń”ÓƵÄŅĒĘ÷ŹĒ_____”£
a.ÉÕ±””””””b.²£Į§°ō””””””c.ĮæĶ²””””””d.ČŻĮæĘæ
(2)ÉĻŹö×°ÖĆÖŠ£¬AŹĒ_____ČÜŅŗ£¬NaHCO3ČÜŅŗæÉŅŌĪüŹÕ_____”£
(3)ÉĻŹö×°ÖĆÖŠ£¬BĪļÖŹŹĒ_____”£°ŃÕāøöŹµŃéµĆµ½µÄĘųĢåŹÕ¼ÆĘšĄ“£¬ÓĆĄ“²ā¶ØCO2µÄ·Ö×ÓĮ棬Čē¹ūBĪļÖŹŹ§Š§£¬²ā¶Ø½į¹ū_____(Ģī”°Ę«øß”±”¢”°Ę«µĶ”±»ņ”°²»ŹÜÓ°Ļģ”±)”£
(4)Ņ»“ĪŠŌ·¹ŗŠÖŠŹÆĄÆ(øß¼¶ĶéĢž)ŗĶCaCO3ŌŚŹ³ĪļÖŠµÄČܳöĮæŹĒĘĄ¼Ū·¹ŗŠÖŹĮæµÄÖø±źÖ®Ņ»£¬²ā¶ØČܳöĮæµÄÖ÷ŅŖŹµŃé²½ÖčÉč¼ĘČēĻĀ£ŗ
¼ōĖ锢³ĘÖŲ”ś½žÅŻČܽā”ś¹żĀĖ”ś²ŠŌüŗęøÉ”śĄäČ“”¢³ĘÖŲ”śŗćÖŲ£¬ĪŖĮĖ½«ŹÆĄÆČܳö£¬Ó¦Ń”ÓƵďŌ¼ĮŹĒ_____£¬Ģ¼ĖįøĘČܳö£¬Ó¦Ń”ÓƵďŌ¼ĮŹĒ_____”£
a.ĀČ»ÆÄĘČÜŅŗ””””””””b.Ļ”“×Ėį
c.Ļ”ĮņĖį””””””””””””d.Õż¼ŗĶé
(5)ŌŚČܳöĮæ²ā¶ØŹµŃéÖŠ£¬ĪŖĮĖ»ńµĆŹÆĄÆŗĶĢ¼ĖįøʵÄ×ī“óČܳöĮ棬ӦĻČČܳö_____£¬ŌŅņŹĒ_______________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ijæĪĶā»ī¶ÆŠĖȤŠ”×éÓūÓĆĻĀĮŠ»ÆѧŹŌ¼ĮŌŚŹµŃéŹŅĄļÖĘČ”Cl2²¢ŃéÖ¤Cl2µÄijŠ©»ÆѧŠŌÖŹ”£ŹµŃéŹŌ¼Į£ŗ3 mol”¤L£1 H2SO4”¢1 mol”¤L£1 NaOHČÜŅŗ”¢MnO2”¢KMnO4”¢ĪüŹÕÉŁĮæSO2µÄNaOHČÜŅŗ”¢ÅØŃĪĖį”¢×ĻÉ«ŹÆČļŹŌŅŗ”¢±„ŗĶNaClČÜŅŗ”¢BaCl2ČÜŅŗ”¢Ę·ŗģČÜŅŗ”£¼×Š”×éÉč¼ĘµÄŹµŃé×°ÖĆĶ¼ČēĻĀ£¬ŌŚBÖŠ¼ÓČėĪüŹÕÉŁĮæSO2µÄNaOHČÜŅŗ£¬DÖŠ¼ÓČė1 mol”¤L£1 NaOHČÜŅŗ”£Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ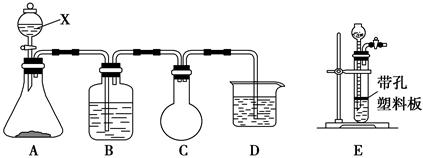
¢ń.£Ø1£©Š“³öŅĒĘ÷XµÄĆū³Ę£ŗ________”£
£Ø2£©Š“³öAÖŠ·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½£ŗ__________________________________£¬
²»ÄÜÓĆE×°ÖĆ“śĢęA×°ÖƵÄŌŅņŹĒ___________________________________”£
£Ø3£©C×°ÖƵÄ×÷ÓĆŹĒ_________________________________________________”£
£Ø4£©¼×Š”×éĶعżøĆŹµŃéŅŖŃéÖ¤Cl2µÄ________£ØĢī”°Ęư׊Ō”±”¢”°»¹ŌŠŌ”±»ņ”°Ńõ»ÆŠŌ”±£©”£
£Ø5£©ŅŅŠ”×éČĻĪŖ¼×Š”×éÉč¼ĘµÄŹµŃé×°ÖĆӊȱĻŻ£¬ĒėÄć°ļÖś¼×Š”×éĶźÉĘøĆ×°ÖĆ”£¼“»¹ŅŖŌŚ________ŗĶ________£ØĢī”°A”±”¢”°B”±”¢”°C”±»ņ”°D”±£©×°ÖĆ¼äĮ¬½ÓŅ»øö×°ÓŠ________µÄĻ“Ęų×°ÖĆ”£
¢ņ.±ūŠ”×éČĻĪŖ·“Ó¦Ņ»¶ĪŹ±¼äŗó£¬B×°ÖĆČÜŅŗ£ØĒæ¼īŠŌ£©ÖŠæĻ¶Ø“ęŌŚCl£”¢OH£ŗĶSO£¬æÉÄÜ»¹ŗ¬ÓŠĘäĖūµÄŅõĄė×Ó”£
ĒėÄćÉč¼ĘŹµŃé°ļÖś±ūŠ”×éĢ½¾æøĆĪüŹÕŅŗÖŠæÉÄÜ“ęŌŚµÄĘäĖūŅõĄė×Ó”£
£Ø1£©Ģį³öŗĻĄķ¼ŁÉč
¼ŁÉč1£ŗÖ»“ęŌŚ________£¬»ņÖ»“ęŌŚ________”£
¼ŁÉč2£ŗæÉÄÜĮ½Õ߶¼________£ØĢī”°“ęŌŚ”±»ņ”°²»“ęŌŚ”±£©”£
£Ø2£©ĒėÉč¼ĘŹµŃéŃéÖ¤¼ŁÉčµÄÕżČ·ŠŌ£ŗ___________________________________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
K3[Fe(C2O4)3]”¤3H2O[Čż²ŻĖįŗĻĢś(¢ó)Ėį¼Ų¾§Ģå]Ņ×ČÜÓŚĖ®,ÄŃČÜÓŚŅŅ“¼,æÉ×÷ĪŖÓŠ»ś·“Ó¦µÄ“߻ƼĮ”£ŹµŃéŹŅæÉÓĆĢśŠ¼ĪŖŌĮĻÖʱø,Ļą¹Ų·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ:Fe+H2SO4 FeSO4+H2”ü
FeSO4+H2ӟ
FeSO4+H2C2O4+2H2O FeC2O4”¤2H2O”ż+H2SO4
FeC2O4”¤2H2O”ż+H2SO4
2FeC2O4”¤2H2O+H2O2+H2C2O4+3K2C2O4 2K3[Fe(C2O4)3]+6H2O
2K3[Fe(C2O4)3]+6H2O
2Mn +5C2
+5C2 +16H+
+16H+ 2Mn2++10CO2ӟ+8H2O
2Mn2++10CO2ӟ+8H2O
»Ų“šĻĀĮŠĪŹĢā:
(1)ĢśŠ¼ÖŠ³£ŗ¬ĮņŌŖĖŲ,Ņņ¶ųŌŚÖʱøFeSO4Ź±»į²śÉśÓŠ¶¾µÄH2SĘųĢå,øĆĘųĢåæÉÓĆĒāŃõ»ÆÄĘČÜŅŗĪüŹÕ”£ĻĀĮŠĪüŹÕ×°ÖĆÕżČ·µÄŹĒ”””””””””””£ 
(2)ŌŚ½«Fe2+Ńõ»ÆµÄ¹ż³ĢÖŠ,ŠčæŲÖĘČÜŅŗĪĀ¶Č²»øßÓŚ40 ”ę,ĄķÓÉŹĒ””””””””””””””;µĆµ½K3[Fe(C2O4)3]ČÜŅŗŗó,¼ÓČėŅŅ“¼µÄĄķÓÉŹĒ”””””””””””””””””£
(3)¾§ĢåÖŠĖłŗ¬½į¾§Ė®æÉĶعżÖŲĮæ·ÖĪö·Ø²ā¶Ø,Ö÷ŅŖ²½ÖčÓŠ:¢Ł³ĘĮæ,¢ŚÖĆÓŚŗęĻäÖŠĶŃ½į¾§Ė®,¢ŪĄäČ“,¢Ü³ĘĮæ,¢Ż””””””””””””””””””””(ŠšŹö“Ė²½²Ł×÷),¢Ž¼ĘĖć”£²½Öč¢ŪČōĪ“ŌŚøÉŌļĘ÷ÖŠ½ųŠŠ,²āµĆµÄ¾§ĢåÖŠĖłŗ¬½į¾§Ė®ŗ¬Įæ””””””””(Ģī”°Ę«øß”±”°Ę«µĶ”±»ņ”°ĪŽÓ°Ļģ”±);²½Öč¢ŻµÄÄæµÄŹĒ”””””””””””””””””””””””””””””””£
(4)¾§ĢåÖŠC2 ŗ¬ĮæµÄ²ā¶ØæÉÓĆĖįŠŌKMnO4±ź×¼ČÜŅŗµĪ¶Ø”£³ĘȔȿ²ŻĖįŗĻĢś(¢ó)Ėį¼Ų¾§Ģåm gČÜÓŚĖ®Åä³É250 mLČÜŅŗ,Č”³ö20.00 mL·ÅČė׶ŠĪĘæÖŠ,ÓĆ0.010 0 mol”¤L-1Ėį»ÆµÄøßĆĢĖį¼ŲČÜŅŗ½ųŠŠµĪ¶Ø”£
ŗ¬ĮæµÄ²ā¶ØæÉÓĆĖįŠŌKMnO4±ź×¼ČÜŅŗµĪ¶Ø”£³ĘȔȿ²ŻĖįŗĻĢś(¢ó)Ėį¼Ų¾§Ģåm gČÜÓŚĖ®Åä³É250 mLČÜŅŗ,Č”³ö20.00 mL·ÅČė׶ŠĪĘæÖŠ,ÓĆ0.010 0 mol”¤L-1Ėį»ÆµÄøßĆĢĖį¼ŲČÜŅŗ½ųŠŠµĪ¶Ø”£
¢ŁĻĀĮŠ²Ł×÷¼°Ėµ·ØÕżČ·µÄŹĒ”””””””£
A.µĪ¶Ø¹ÜÓĆÕōĮóĖ®Ļ“µÓŗó,¼“æÉ×°Čė±ź×¼ČÜŅŗ
B.×°Čė±ź×¼ČÜŅŗŗó,°ŃµĪ¶Ø¹Ü¼ŠŌŚµĪ¶Ø¹Ü¼ŠÉĻ,ĒįĒį×Ŗ¶Æ»īČū,·Å³öÉŁĮæ±ź×¼Ņŗ,Ź¹¼ā×ģ³äĀśŅŗĢå
C.½Ó½üÖÕµćŹ±,ŠčÓĆÕōĮóĖ®³åĻ“Ęæ±ŚŗĶµĪ¶Ø¹Ü¼ā¶ĖŠü¹ŅµÄŅŗµĪ
¢ŚÓŠĶ¬Ń§ČĻĪŖøƵĪ¶Ø¹ż³Ģ²»ŠčŅŖÖøŹ¾¼Į,ÄĒĆ“µĪ¶ØÖÕµćµÄĻÖĻóĪŖ””””””””””””””””,Čō“ļµ½µĪ¶ØÖÕµćĻūŗÄøßĆĢĖį¼ŲČÜŅŗV mL,ÄĒĆ“¾§ĢåÖŠĖłŗ¬C2 µÄÖŹĮæ·ÖŹżĪŖ””””””””(ÓĆŗ¬V”¢mµÄŹ½×Ó±ķŹ¾)”£
µÄÖŹĮæ·ÖŹżĪŖ””””””””(ÓĆŗ¬V”¢mµÄŹ½×Ó±ķŹ¾)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
¶žĀČ»Æ¶žĮņ£ØS2Cl2£©ŌŚ¹¤ŅµÉĻÓĆÓŚĻš½ŗµÄĮņ»Æ”£ĪŖŌŚŹµŃéŹŅŗĻ³ÉS2Cl2£¬Ä³»Æѧъ¾æŠŌѧĻ°Š”×é²éŌÄĮĖÓŠ¹Ų׏ĮĻ£¬µĆµ½ČēĻĀŠÅĻ¢£ŗ
¢Ł½«øÉŌļµÄĀČĘųŌŚ110”ꔫ140”ęÓėĮņ·“Ó¦£¬¼“æɵĆS2Cl2“ÖĘ·”£
¢ŚÓŠ¹ŲĪļÖŹµÄ²æ·ÖŠŌÖŹČēĻĀ±ķ£ŗ
| ĪļÖŹ | ČŪµć/”ę | ·Šµć/”ę | »ÆѧŠŌÖŹ | ||
| S | 112.8 | 444.6 | ĀŌ | ||
| S2Cl2 | £77 | 137 |
|

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
Ń”ÓĆŹŹµ±×°ÖĆ”¢ŹŌ¼ĮŗĶ·½·ØæÉÖʱø֊ѧ»Æѧ֊µÄ¼øÖÖ³£¼ūĘųĢ唣ĒėĢīŠ“±ķÖŠµÄæÕøń£Ø“Ó¢Ł”«¢āÖŠŃ”Ōń£¬ĢīŠņŗÅ£©£ŗ
| ŹµŃé | ĘųĢå | ĖłÓĆ×°ÖĆ | ĘųĢåŠŌÖŹ | øÉŌļøĆĘųĢåŃ”ÓƵďŌ¼Į |
| £Ø1£© | | ¢Ū | ĘäĖ®ČÜŅŗĻŌ¼īŠŌ | |
| £Ø2£© | | | 1mol×ī¶ąÄÜÓė2molH2·“Ó¦ | ¢ā |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠŹµŃé²Ł×÷²»ÄÜ“ļµ½Ō¤ĘŚŹµŃéÄæµÄµÄŹĒ
| | ŹµŃéÄæµÄ | ŹµŃé²Ł×÷ |
| A | ¼ų±šŅŅĖįŅŅõ„ŗĶŅŅĖį | ·Ö±š¼ÓČė±„ŗĶNa2CO3ČÜŅŗ |
| B | ±Č½ĻFeŗĶCuµÄ½šŹō»ī¶ÆŠŌ | ·Ö±š¼ÓČėÅØĻõĖį |
| C | ±Č½ĻH2OŗĶŅŅ“¼ÖŠōĒ»łĒāµÄ»īĘĆŠŌ | ·Ö±š¼ÓČėÉŁĮæNa |
| D | ±Č½ĻI2ŌŚH2OŗĶCCl4ÖŠµÄČܽā¶Č | ĻņI2Ė®ÖŠ¼ÓČėCCl4£¬Õńµ“ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com