
����Ŀ��(1)һ�������£�CO2��1.0mol��L-1NaOH��Һ��ַ�Ӧ�ų����������±���
��Ӧ��� | CO2�����ʵ���/mol | NaOH��Һ�����/L | �ų�������/kJ |
1 | 0.5 | 0.75 | X |
2 | 1.0 | 2.00 | y |
��������CO2��NaOH��Һ��Ӧ����NaHCO3���Ȼ�ѧ����ʽΪ_____________________��
(2)�ý�̿��ԭNO�ķ�ӦΪ��2NO(g)+C(s)![]() N2(g)+CO2(g)�����ݻ���Ϊ1L�ļס��ҡ����������������зֱ���������Ľ�̿��һ������NO����ø�������n(NO)�淴Ӧʱ��t�ı仯������±���
N2(g)+CO2(g)�����ݻ���Ϊ1L�ļס��ҡ����������������зֱ���������Ľ�̿��һ������NO����ø�������n(NO)�淴Ӧʱ��t�ı仯������±���
�¶� t/min | 0 | 40 | 80 | 120 | 160 |
��(673K) | 2.00 | 1.50 | 1.10 | 0.80 | 0.80 |
��(T) | 2.00 | 1.45 | 1.00 | 1.00 | 1.00 |
��(673K) | 1.00 | 0.80 | 0.65 | 0.53 | 0.45 |
�ټ������У�0��40min����NO��Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(NO)=____________��
�ڸ÷�Ӧ�ġ�H__________(�����)
a������0 b��С��0 c������0 d������ȷ��
�۱������ﵽƽ��ʱ��NO��ת����Ϊ__________________��
(3)298Kʱ��NH3��H2O�ĵ��볣��Kb=2��10-5��H2CO3�ĵ��볣��Kal=4��10-7��Ka2=4��10-11����NH4HCO3��Һ�У�c(NH4+)__________c(HCO3-)(����>������<������=��)����ӦNH4++HCO3-+H2O![]() NH3��H2O+H2CO3��ƽ�ⳣ��K����ֵ(�ÿ�ѧ��������ʾ)Ϊ_____��
NH3��H2O+H2CO3��ƽ�ⳣ��K����ֵ(�ÿ�ѧ��������ʾ)Ϊ_____��
���𰸡�CO(g)+NaOH(aq)��NaHCO3(aq)����H=(y-4x)kJ/mol 0.0125molL-1min-1 b 60% > 1.25��10-3
��������
(1)�ɱ������ݣ���д�����������Ȼ�ѧ����ʽ��
��Ӧ1��2CO2(g)+3NaOH(ag)==Na2CO3(ag)+NaHCO3(ag)+H2O(l) H1= -4xkJ/mol
��Ӧ2��CO2(g)+2NaOH(ag)==Na2CO3(ag) +H2O(l) H2= -ykJ/mol
�ɸ�˹���ɣ�����Ӧ1-��Ӧ2�����ɵõ���������CO2��NaOH��Һ��Ӧ����NaHCO3���Ȼ�ѧ����ʽ��
(2)�ټ������У�0��40min����NO��Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(NO)=![]() ��
��
�ڼ����ҽ��жԱȣ��Ӵ�ƽ���ʱ�俴��������ʱ��̣������T>673K��ƽ��ʱ����n(NO)�Ӷ��ó������¶ȣ�ƽ�������ƶ��Ľ��ۡ�
����Ϊ��Ӧǰ�������������ȣ������¶���ͬʱ���ı�ѹǿƽ�ⲻ�����ƶ����������NO��ת������ͬ�����ڱ���NO��ƽ��Ũ��δ֪�����Կ����ü���������ﵽƽ��ʱ��NO��ת���ʡ�
(3)�Ա�NH3��H2O��HCO3-�ĵ��볣�����Ӷ��ó����ߵ�ˮ�ⳣ����ϵ���ɴ��Ƴ���NH4HCO3��Һ�У�c(NH4+)��c(HCO3-)�Ĵ�С��ϵ����ӦNH4++HCO3-+H2O![]() NH3��H2O+H2CO3��ƽ�ⳣ��K=
NH3��H2O+H2CO3��ƽ�ⳣ��K=![]() =
=![]() =
=![]() ���������ݼ������K��
���������ݼ������K��
(1)�ɱ������ݣ���д�����������Ȼ�ѧ����ʽ��
��Ӧ1��2CO2(g)+3NaOH(ag)==Na2CO3(ag)+NaHCO3(ag)+H2O(l) H1= -4xkJ/mol
��Ӧ2��CO2(g)+2NaOH(ag)==Na2CO3(ag) +H2O(l) H2= -ykJ/mol
�ɸ�˹���ɣ�����Ӧ1-��Ӧ2�����ɵõ���������CO2��NaOH��Һ��Ӧ����NaHCO3���Ȼ�ѧ����ʽΪCO(g)+NaOH(aq)��NaHCO3(aq)����H=(y-4x)kJ/mol����Ϊ��CO(g)+NaOH(aq)��NaHCO3(aq)����H=(y-4x)kJ/mol��
(2)�ټ������У�0��40min����NO��Ũ�ȱ仯��ʾ��ƽ����Ӧ����v(NO)=![]() = 0.0125molL-1min-1����Ϊ��0.0125molL-1min-1��
= 0.0125molL-1min-1������0.0125molL-1min-1��
�ڼ����ҽ��жԱȣ��Ӵ�ƽ���ʱ�俴��������ʱ��̣������T>673K��ƽ��ʱ����n(NO)�Ӷ��ó������¶ȣ�ƽ�������ƶ����÷�Ӧ�ġ�H<0����ѡb����Ϊ��b��
����Ϊ��Ӧǰ�������������ȣ������¶���ͬʱ���ı�ѹǿƽ�ⲻ�����ƶ����������NO��ת������ͬ�����ڱ���NO��ƽ��Ũ��δ֪�����Կ����ü���������ﵽƽ��ʱ��NO��ת����Ϊ![]() =60%����Ϊ��60%��
=60%������60%��
(3) 298Kʱ��NH3��H2O�ĵ��볣��Kb=2��10-5��H2CO3�ĵ��볣��Ka2=4��10-11������NH4+��ˮ��������HCO3-������NH4HCO3��Һ�У�c(NH4+)>c(HCO3-)����ӦNH4++HCO3-+H2O![]() NH3��H2O+H2CO3��ƽ�ⳣ��K=
NH3��H2O+H2CO3��ƽ�ⳣ��K=![]() =
=![]() =
=![]() =
=![]() =1.25��10-3������>��1.25��10-3��
=1.25��10-3������>��1.25��10-3��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и�����ָ���ı�ֵ��2��1���� ( )
A. C2H4�������г��ȼ�����ɵ�CO2��H2O�����ʵ���֮��
B. K2S��Һ��c(K+)��c(S2-)֮��
C. Na2O2�������������������ӵ����ʵ���֮��
D. CsCl���������������ӵ���λ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������°����ܱ�������������N2�����������ô˷�����ܵ��Ƿ�й©������ij̽��С����ʵ���Ҷ�����������Ӧ������̽�����ش��������⡣
�������Ʊ�
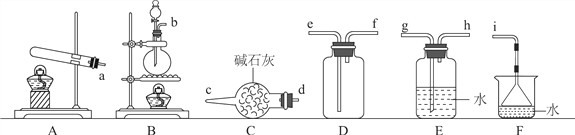
��1�������ķ���װ�ÿ���ѡ����ͼ�е�________�����д��ĸ������Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��2�����ռ�һƿ����İ�����ѡ����ͼ�е�װ�ã�����������д����ӿڵ�����˳����װ�ùܿڡ�________����Сд��ĸ����
�������백���ķ�Ӧ
�����£����ռ����İ�������ͼ��ʾװ�ý���ʵ�飨ʵ��ǰk1��k2�رգ���
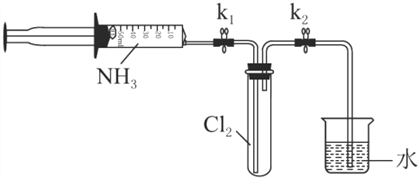
��3����k1�������ƶ�ע�������������Թ���ע��Լ3������������İ������ر�k1���ָ����¡��Թ��пɹ۲쵽��������________��������Ӧ�Ļ�ѧ����ʽΪ________��
��4���ٴ�k2���ɹ۲쵽��������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ijͭ��ʯ����Ҫ�ɷ���Cu2O��������������Al2O3��Fe2O3��SiO2��ijѧϰС��ģ�⻯��������������������Ʊ���ͭ��

��֪��Cu2O + 2 H�� = Cu + Cu2�� + H2 O
�ش��������⣺
(1)ʵ�������У�����ͭ��ʯ�����Ŀ����______________________________��
(2)����1�к��н϶��ͭ���ᴿ����1ʱ��Ӧ�����ӷ���ʽΪ_______________________________��
(3)��Һ1����Ԫ�صĴ�����ʽΪ______________(�����ӷ���)����������ӵij����Լ�Ϊ________________��
(4)д����������ʱ����ͭ�Ļ�ѧ����ʽ��______________________________��
(5)����������������У���ͭ����ӵ�Դ��___________�������������ĵ缫��ӦʽΪ__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ��HCl�ӳɿ��ܷ�����Ӧ�ٺ͢ڣ��������뷴Ӧ������ͼ��ʾ������˵����ȷ����
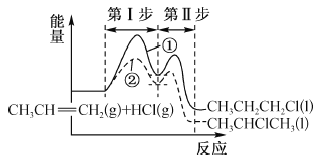
A. CH3CHClCH3 �� CH3CH2CH2Cl �ȶ�
B. ��Ӧ�ٵĵ�I����ڢ��ų�����
C. ��Ӧ�ڵĵ�I���ȵڢ�Ӧ���ʿ�
D. ��ܷ�Ӧ�ٱȢڵĴ�Ӧ�ٸ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪�ס��ҡ����������ʾ�����ͬ��Ԫ��X����ת����ϵ���£�
![]()
����˵���������
A.��AΪNaOH��Һ����Ϊ��ɫ��������X����Ϊ�����ڽ���Ԫ��
B.��AΪ���ᣬXΪ����Ԫ�أ�������ҷ�Ӧ�����ɱ�
C.��AΪ����������ͨ��״����Ϊ����ɫ���壬�����Ϊ�ǽ�������
D.����ΪNaHCO3������������CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������(Na2S2O3)�㷺���ڵ�ơ�����Ƥ���֯ƷƯ������ȼ��ȡ�ijʵ��С���ͬѧ��Na2S��SO2Ϊԭ���Ʊ�Na2S2O3���ش��������⣺
(1)�Ʊ�Na2S��Һ
��Na2S��Һ�ʼ��ԣ�ԭ����____________________________(�����ӷ���ʽ��ʾ)��
�ڹ�ҵƷ�����г���������Na2SO4��Na2CO3������д��������ǽ���������ˮ��Ȼ�����������_____________(�ѧʽ)��Һ��ֽ��貢���ˣ��پ��Ƶ�Na2S��Һ��
(2)������ͼװ���Ʊ�Na2S2O3
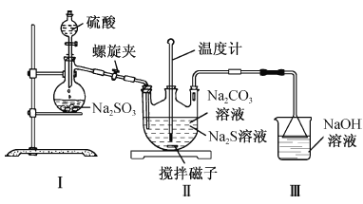
��װ�â��з�Ӧ�Ļ�ѧ����ʽΪ___________________________________��
��ʵ������д������в��������������ʵ���λ�ã�һ����ʹ��Ӧ������SO2����Ͼ��ȵ�ͨ��Na2S��Na2CO3��Һ�У���һ�����������______________________________��
��������ƿ������Na2S2O3�Ļ�ѧ����ʽΪ��____________________________________��װ�â��������_________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��д���������ʵĽṹ��ʽ��
��1��2��4��6��������5���һ����飺________________________________��
��2��3������1����Ȳ��______________________________��
��3��1��4�����ϩ��______________________________��
��4������ϩ��___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ڷ�Ӧ2HI(g) ![]() H2(g) +I2(g),���������ܹ�˵������ƽ��״̬����
H2(g) +I2(g),���������ܹ�˵������ƽ��״̬����
A. ����������ɫ���ٱ仯
B. �¶Ⱥ����һ��ʱ��������ѹǿ���ٱ仯
C. lmolH-H�����ɵ�ͬʱ��2molH-I������
D. �����ʵ����ʵ���Ũ��֮��Ϊ2��1��1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com