
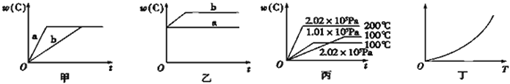
�ٵ�a=b+cʱ��B�����ʵ�������_____________��
�ڵ�a��b+cʱ��A�����ʵ�������_____________��
�۵�a��b+cʱ��A��ת����_____________��
��2���������ʵ�����A��B�������2 L���ܱ������У��������·�Ӧ:3A(g)+B(g)=== xC(g)+2D(g)������5 min�����D��Ũ��Ϊ0.5 mol��L-1��c(A)��c(B)=3��5��C�ķ�Ӧ������0.1 mol��L-1��min-1��A��5 minĩ��Ũ����_____________��B��ƽ����Ӧ������_____________��x��ֵ��_____________��
��1���ٲ��� �ڼ�С �ۼ�С
��2��0.75 mol��L-1 0.05 mol��L-1��min-1 2
���������ڹ̶��ݻ����ܱ������л�ѧƽ��:aA��g��![]() bB��g��+cC��g��,���¶Ȳ���������£����������ٳ���һ������A���ʣ����´ﵽƽ��ʱ����a=b+cʱ��B�����ʵ����������䣬��?a��b+c?ʱ��A�����ʵ���������С����a��b+cʱ��A��ת���ʼ�С�������ʵ�����A��B�����2 L���ܱ������У��������·�Ӧ��3A��g��+B��g��
bB��g��+cC��g��,���¶Ȳ���������£����������ٳ���һ������A���ʣ����´ﵽƽ��ʱ����a=b+cʱ��B�����ʵ����������䣬��?a��b+c?ʱ��A�����ʵ���������С����a��b+cʱ��A��ת���ʼ�С�������ʵ�����A��B�����2 L���ܱ������У��������·�Ӧ��3A��g��+B��g��![]() xC��g��+2D��g����A��5 minĩ��Ũ����0.75 mol��L-1��B��ƽ����Ӧ������0.05 mol��L-1��min-1��x��ֵ��2��
xC��g��+2D��g����A��5 minĩ��Ũ����0.75 mol��L-1��B��ƽ����Ӧ������0.05 mol��L-1��min-1��x��ֵ��2��


 �����Ļ�������ҵϵ�д�
�����Ļ�������ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ٵ�a=b+cʱ��B�����ʵ�������________________________________________��
�ڵ�a��b+cʱ��A�����ʵ�������________________________________________��
�۵�a��b+cʱ��A��ת����________________________________________________��
(2)�������ʵ�����A��B�������![]() xC(g)+2D(g)������5 min�����D��Ũ��Ϊ0.5 mol��L-1��c(A)��c(B)=3��5��C�ķ�Ӧ������0.1 mol��L-1��min-1��A��5 minĩ��Ũ����____________��B��ƽ����Ӧ������____________��x��ֵ��____________��
xC(g)+2D(g)������5 min�����D��Ũ��Ϊ0.5 mol��L-1��c(A)��c(B)=3��5��C�ķ�Ӧ������0.1 mol��L-1��min-1��A��5 minĩ��Ũ����____________��B��ƽ����Ӧ������____________��x��ֵ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ٵ�a=b+cʱ��B�����ʵ�������________________________________________��
�ڵ�a��b+cʱ��A�����ʵ�������________________________________________��
�۵�a��b+cʱ��A��ת����________________________________________________��
(2)�������ʵ�����A��B�������![]() xC(g)+2D(g)������5 min�����D��Ũ��Ϊ0.5 mol��L-1��c(A)��c(B)=3��5��C�ķ�Ӧ������0.1 mol��L-1��min-1��A��5 minĩ��Ũ����____________��B��ƽ����Ӧ������____________��x��ֵ��____________��
xC(g)+2D(g)������5 min�����D��Ũ��Ϊ0.5 mol��L-1��c(A)��c(B)=3��5��C�ķ�Ӧ������0.1 mol��L-1��min-1��A��5 minĩ��Ũ����____________��B��ƽ����Ӧ������____________��x��ֵ��____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.A��B��C��Ũ��֮��Ϊ1��3��2
B.��λʱ��������n mol A��ͬʱ����3n mol B
C.��λʱ��������n mol A��ͬʱ����2n mol C
D.���������ܶȲ���
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com