



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
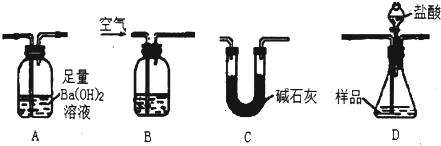
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ��ͷ�д����ѧ�߶���ѧ����ĩ���ƻ�ѧ�Ծ����������� ���ͣ������
�밴Ҫ��������и�����գ�
��1��AlCl3��ˮ��Һ�� ����ᡱ�����С�����ԣ�����ʱ��pH 7�����������������������ԭ���ǣ������ӷ���ʽ��ʾ���� ��ʵ���������� AlCl3��Һʱ������ AlCl3����������Ũ�����У�Ȼ����������ˮϡ�͵������Ũ�ȣ��� ����ٽ��������ơ�����ˮ�⡣��AlCl3��Һ���ɡ����գ����õ�����Ҫ��������� ���ѧʽ����
��2���ڴ�����Һ�е����̪����Һ��졣���ڸ���Һ���ٵ���������Ȼ�����Һ�����۲쵽��������___________________________________________����ԭ���ǣ������ӷ���ʽ�ͼ�Ҫ������˵������
__________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014��㶫ʡ��ͷ�и߶���ѧ����ĩ���ƻ�ѧ�Ծ��������棩 ���ͣ������
�밴Ҫ��������и�����գ�
��1��AlCl3��ˮ��Һ�� ����ᡱ�����С�����ԣ�����ʱ��pH 7�����������������������ԭ���ǣ������ӷ���ʽ��ʾ���� ��ʵ���������� AlCl3��Һʱ������ AlCl3����������Ũ�����У�Ȼ����������ˮϡ�͵������Ũ�ȣ��� ����ٽ��������ơ�����ˮ�⡣��AlCl3��Һ���ɡ����գ����õ�����Ҫ��������� ���ѧʽ����
��2���ڴ�����Һ�е����̪����Һ��졣���ڸ���Һ���ٵ���������Ȼ�����Һ�����۲쵽��������___________________________________________����ԭ���ǣ������ӷ���ʽ�ͼ�Ҫ������˵������
__________________________________________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com