
����Ŀ����ȩ�ܹ��������з�Ӧ��
Cu2O+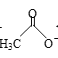
![]()
![]()
![]()
(1)Mn2���Ļ�̬�����Ų�ʽΪ____��
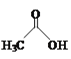
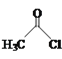
(2)�Ȼ�����(SOCl2)���л��ϳ�����Ҫ���Ȼ�������SOCl2��Ϊ�ȵ�����������ӵĻ�ѧʽΪ___��
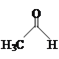
(3)CH3CHO��������ԭ�ӵĹ���ӻ�������____��
(4)����ķе�(117.9 ��)����ȩ�ķе�(20.8 ��)�ߵ���Ҫԭ����____��
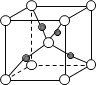
(5)�����ǿռ乹�ͣ�[Cu(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ____��
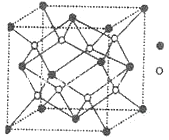
(6)��ͼ��ʾCu2O�ľ�����Cu������λ����____��
���𰸡�[Ar]3d5 SO32-��ClO3- sp2 ������Ӽ�����������ȩ���Ӽ䲻������� 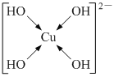 ��
�� 2
2
��������
(1)���ݺ�����ӵ��Ų�����Mn2���Ļ�̬�����Ų�ʽΪ[Ar]3d5��
(2)���ݵȵ�����Ķ��壬ԭ����Ŀ��ͬ���۵�����ĿҲ��ͬ�ķ��ӣ����ӣ���Ϊ�ȵ����壬��SOCl2��Ϊ�ȵ�����������ӵĻ�ѧʽΪSO32-��ClO3-��
(3)CH3CHO��������ԭ�ӵļ۲���Ӷ�����3�����ӻ������ҲΪ3�����ӻ�������sp2��
(4)�������ȩ��Ϊ���Ӿ��壬������Ӽ�������������ȩ����֮�䲻���������������ķе�ϸߣ�
(5)��������Cu2+�ṩ�չ����4��OH���е���ԭ���ṩ�µ��Ӷԣ��γ���λ�����ʲ����ǿռ乹�ͣ�[Cu(OH)4]2���Ľṹ����ʾ��ͼ��ʾΪ ��
�� ��
��
(6)���ݾ����ṹ��ÿ��Cu����Χ����Ⱦ�������O-����Cu������λ����2��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���Ʊ��Ʋ�������Fe3O4����ģ��������ˮ����Ԫ�ص���Ҫʵ���������£�

��֪����CaO2��������Һ�е�FeCl2����Ӧ����Fe(OH)3��Fe3����
�ڲ��ӵ�Ca2��Ƕ��Fe3O4�У�ϴ��ʱ����ʧ������ʱ���γ�Ca3(PO4)2�ȳ�����
����Һ��pH���������������������Ӱ�졣pHԽ�ߣ��������������Խ�ࣻ pHԽ�ͣ��������������Խ�ࡣ
��1����FeCl2��FeCl3�����Һ�еμ�NaOH��Һ��һ�������·�Ӧ����Fe3O4�������ӷ���ʽΪ___________��
��2����������pH��11������������70�������½��У����˵ļ��ȷ�ʽΪ________��Ϊ��߹�����Ч�������ɲ�ȡ�Ĵ�ʩΪ_______________��
��3����Ԫ�ص�����Ч����H3PO4ˮ��Һ�к������ֲַ�������pH�Ĺ�ϵ�ֱ���ͼ1��ͼ2��ʾ��

�ٲ������KH2PO4��Һģ���ˮ���������������(pH > 2)����Ԫ���������ϴ�ԭ���ǣ�pHԽ�ͣ��������������������Խ�࣬���������������ӣ�___________________
�ڲ������������ȡ�����ü�Һ���������ס���ϱ������ݣ������Ʋ�������Fe3O4������������������������ȵ������У�________��
��ͬ���������������������������Ƚ�
������ | ����Ʒ | ��Fe3O4 | �մɲ��� |
������/mg��g��1 | 24.1 | 5.0 | 12.5 |
��4������ƴӲ����Ӧ���������ƿ�л�ȡ�Ʋ�������Fe3O4��Ʒ��ʵ�鷽�����ô��������������Һ���룬______________����ɸ��ɸ�ֵõ���Ʒ (ʵ������ʹ�����Լ��������У�����ˮ����ˮ�Ҵ���pH�ơ��в�������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����л�ѧ����ʽ�����ӷ���ʽ��ȷ����
A.������ˮ��Ӧ��2![]() +Br2
+Br2![]() 2
2![]()
B.1-�ȱ����м�������������Һ�����ȣ�CH3CH2CH2Cl��NaOH��CH3CH=CH2����NaCl��H2O
C.��������Һ��ͨ������CO2��C6H5O��+H2O+CO2��C6H5OH+HCO3��(C6H5-��������)
D.ʵ������ȡ��Ȳ�ķ�Ӧ��CaC2+H2O �� CaO + CH��CH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������⾫����һ���ܼ���֬�����������ҩƷ���к�Σ�յĸ����ã����������ɲ��룬���������ಡ�����й������ֳ������⾫��˵������ȷ����
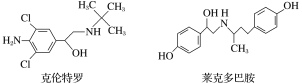
A.���������ӵķ���ʽΪC12H19ON2Cl2
B.���������ܷ����ӳɡ���������ȥ�ȷ�Ӧ
C.�����������˶�Ͱ�������FeCl3��Һ����
D.���˶�Ͱ�������NaOH��Һ��Ӧ��������Ļ�ѧʽΪC18H20NO3Na3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��F��G��H��IΪԭ������������ǰ4���ڵ�Ԫ�أ���֪Aԭ�ӵ����������Ǵ�����������2����DΪ���ڱ��е縺������Ԫ�أ�F��Cλ��ͬһ���壬E��G�����ڱ���λ����������������Ԫ����������������Ԫ�ض��ǽ�����HΪӦ����㷺�Ľ�����I�������ڱ��еĵڶ����塣��ش��������⣺
��1��B���ʷ�����![]() ��
��![]() ����Ŀ��Ϊ____��B��C��D��һ��������С�����˳��Ϊ________����дԪ�ط��ţ���
����Ŀ��Ϊ____��B��C��D��һ��������С�����˳��Ϊ________����дԪ�ط��ţ���
��2��HԪ��ԭ�ӵļ۲�����Ų�ʽΪ____��H3�����ӿ���������Ԫ���е������γɵ�ij����������Ѫ��ɫ������������������A��IԪ���е�ijЩԭ���γɵķ��ӻ�Ϊ�ȵ����壬���ַ��ӵĻ�ѧʽΪ______��дһ�ּ��ɣ����÷�������ԭ�ӹ�����ӻ�����Ϊ_______���������ӿռ乹��Ϊ_______��
��3��D��G�����γ���ͼ�ľ�������ڵ��ʾ����Ԫ��_______����дԪ�ط��ţ�����Ԫ��ԭ���ڸþ����е���λ��Ϊ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮ�Ǿ����Դ���⣬�ں�ˮ�������ۺ����÷��棬�����λ��ȫ��ǰ�С��Ӻ�ˮ����ȡʳ�κ���Ĺ�����ͼ��
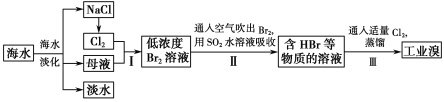
��1�����оٺ�ˮ���������ַ�����___��___��
��2����NaCl��Һ���е�⣬�ڵ����п�ֱ�ӵõ��IJ�Ʒ��H2��___��___��
��3����������ѻ��Br2����������ֽ�Br2��ԭΪBr-����Ŀ����___��
��4���������SO2ˮ��Һ����Br2�������ʿɴ�95%���йط�Ӧ�����ӷ���ʽΪ��___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
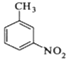
����Ŀ���������ױ���ҽҩ��Ⱦ�ϵȹ�ҵ��һ����Ҫ�л��м��壬������Ũ����Ϊ��������Ũ����Ϊ������ͨ���ױ���������Ӧ�Ʊ���
![]()
![]()
![]() +
+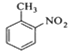 +
+
һ���µ��Ʊ��������ױ���ʵ�鷽���ǣ��Է�������Ϊ������������NaHSO4Ϊ����(��ѭ��ʹ��)����CCl4��Һ�У�����������(����ˮ����)��45�淴Ӧ1h ����Ӧ�������ˣ���Һ�ֱ���5% NaHCO3��Һ��ˮϴ�����ԣ��پ������ᴿ�õ��������ױ���
(1)����ʵ���й��˵�Ŀ����___________��
(2)��Һ�ڷ�Һ©����ϴ�Ӿ��ú��л��㴦��________��(����������'����)��
(3)5% NaHCO3��Һϴ�ӵ�Ŀ����__________
(4)���и����˴������༰�����Լױ�������ӦӰ���ʵ������
���� | n(����)/n(�ױ�) | ���������и����칹����������(%) | �ܲ���(%) | ||
�������ױ� | �������ױ� | �������ױ� | |||
ŨH2SO4 | 1.0 | 35.6 | 60.2 | 4.2 | 98.0 |
1.2 | 36.5 | 59.5 | 4.0 | 99.8 | |
NaHSO4 | 0.15 | 44.6 | 55.1 | 0.3 | 98.9 |
0.25 | 46.3 | 52.8 | 0.9 | 99.9 | |
0.32 | 47.9 | 51.8 | 0.3 | 99.9 | |
0.36 | 45.2 | 54.2 | 0.6 | 99.9 | |
��NaHSO4���Ʊ��������ױ�ʱ��������ױ���������ʵ���֮��Ϊ_______________��
���ɼױ������õ��ĸ��ֲ���ĺ�����֪���ױ�������Ӧ���ص���_________________��
����Ũ������ױ�������ȣ�NaHSO4���ױ��������ŵ���_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����úֱ����Ϊȼ��ȼ�գ�������Ч�ʽϵͣ��Ҳ����̳���������������������ʣ�������صĻ�����Ⱦ��ú�ĸ��������ú�������ʡ�������Ҫ����ԭ�ϡ�������Ⱦ���ŷ�������Ч��ʩ֮һ��ij��ѧѧϰС����ʵ����������̽��ú����������װ����ͼ��ʾ����ش��й����⣺

(1)ú�����������_______��
(2)ʢ����ˮ���ձ���������__________________
(3)ʵ�������дְ�ˮ���ɵIJ�����___
(4)��֪CO����ʹ��ˮ��ɫ�������Ӿ�֧�Թ�֧�ܿڴ��ݳ�������ͨ����ˮ�У�������ˮ��ɫ����˵��ú�ĸ��������_______________________
(5)��ȼβ�����������ɫΪ________________________
(6)��ú�����з���������ױ������ױ���ʵ�鷽����_________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������H��һ�ֹ�������м��塣�������ɷ��㻯����A�Ʊ�H��һ�ֺϳ�·�����£�

��֪����RCHO+CH3CHO ![]() RCH=CHCHO+H2O
RCH=CHCHO+H2O
��![]()
�ش���������
(1)B��������������Ϊ___________��
(2)��B����C��E����F�ķ�Ӧ���ͷֱ�Ϊ___________��___________��
(3)D�Ľṹ��ʽΪ___________��
(4)��A����B�Ļ�ѧ����ʽΪ___________��
(5)���㻯����X��F��ͬ���칹�壬X���뱥��̼��������Һ��Ӧ�ų�CO2����˴Ź���������ʾ��4�ֲ�ͬ��ѧ�������⣬�������Ϊ6��2��1��1������Ҫ���X�Ľṹ��___________�֣�д������һ�ֽṹ��ʽ___________��
(6)��������֪ʶ����������Ϣ��д�����Ҵ�Ϊԭ�Ϻϳ� CH3CH2CH2COOH�ĺϳ�·��(���Լ�����)(�ϳ�·��ʾ�����£�CH2=CH2![]() CH3CH2Br
CH3CH2Br![]() CH3CH2OH ______________________________________________��
CH3CH2OH ______________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com