
̼����ĺ���Ӱ��������ܡ�̼��������һ�ֲⶨ�����ǽ������е�̼����ת��Ϊ���壬���ò�̼������װ�ý��вⶨ��
(1)����װ��A���ڸ����½�x g�����е�̼����ת��ΪCO2��SO2��

������a�ijɷ���______��
��������������FeS��ʽ���ڣ�A�з�Ӧ��
3FeS��5O2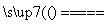 1________��3________��
1________��3________��
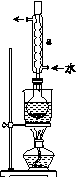
(2)������aͨ�����װ����(��ͼ)�����õζ����ⶨ��ĺ�����
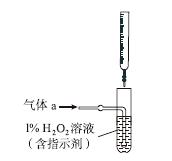
��H2O2����SO2�Ļ�ѧ����ʽ��__________________��
����NaOH��Һ�ζ����ɵ�H2SO4������z mL NaOH��Һ��������1 mL NaOH��Һ�൱���������Ϊy g����ø������������������________��
(3)������aͨ���̼װ����(��ͼ)�������������ⶨ̼�ĺ�����
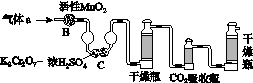
������aͨ��B��C��Ŀ����________________________________________��
�ڼ��������̼������������Ӧ������������_______________________________________________________��
(1)��O2��SO2��CO2����Fe3O4��SO2��
(2)��H2O2��SO2===H2SO4����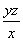 ��
��
(3)���ų�SO2��CO2�ⶨ�ĸ��š�������CO2ǰ��������ƿ������
[����] (1)�ٸ����������գ�̼����ת��Ϊ������̼�Ͷ���������������ɷ�ΪCO2��SO2��O2��������Ԫ�صĴ�����ʽΪFeS�����ݸ����Ļ�ѧ��������3���������ΪSO2������������غ�ȷ��1���������ΪFe3O4����ѧ����ʽΪ3FeS��5O2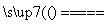 Fe3O4��3SO2��
Fe3O4��3SO2��
(2)��H2O2��SO2��Ӧ�Ļ�ѧ����ʽΪH2O2�� SO2=== H2SO4���ڸ�������1 mL������������Һ�൱������y g��������z mL������������Һ�൱�ں�����Ϊzy g�������������Ϊ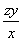 ��
��
(3)�����мȺ��ж��������ֺ��ж�����̼���ⶨ������̼ǰ�����ȥ������������ţ�����B��Cװ�����ڳ�ȥ������̼�еĶ������ⶨ̼�ĺ������ⶨ������̼�����������Ҫ�ⶨ�����������ն�����̼װ��(������̼����ƿ)ǰ�������(������ֵΪ������̼������)��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͬѧ����100 mL 3.6 mol/L��ϡ���ᡣ
(1)������18 mol/L��Ũ����������Һ����Ҫ�õ�Ũ��������Ϊ____________mL����ѡ������ƿ�Ĺ��Ϊ____________mL��
(2)��ͬѧ�����Ʋ��裺��ȡŨ���ᣬС�ĵص���ʢ����������ˮ���ձ��У�������ȣ�����ȴ�����º�ת�Ƶ�100 mL����ƿ�У�������������ˮ���ձ�������ϴ��2��3�Σ�ÿ��ϴ��ҺҲת�Ƶ�����ƿ�У�Ȼ��С�ĵ�������ƿ�м�������ˮ���̶��ߣ����ݣ�����ƿ�����������µߵ�ҡ�ȡ�
��ϴ�Ӳ����У���ϴ���ձ����ϴҺҲע������ƿ����Ŀ����_____________________��
�ڶ��ݵ���ȷ�����Ǽ���������ˮ����̶�����________ʱ������________��ˮ����Һ����ʹ���̶������С�
���ý�ͷ�ι�������ƿ�м�ˮʱ����С��Һ�泬���˿̶ȣ������ķ�����________(�����)��
A����������Һ�壬ʹ��Һ����̶�������
B��С�ļ�������ƿ����������ʹ��Һ����̶�������
C�����������һ������Ũ����
D����������
(3)����ʱ���в����ᵼ��������ҺŨ��ƫ�ߵ���________��
A��ת��ʱ��������Һ����
B������ʱ���Ӷ�ȡ�̶�
C������ƿ������ˮϴ����δ����
D������ʱҺ�泬���˿̶� ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
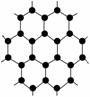 2010��ŵ��������ѧ�����ڱ����״ΰ��������ʯī�Ŀ�ѧ�ҡ�����ʯī��Ϊʯīϩ�����֡�ֻ��һ��̼ԭ�Ӻ��̼��Ƭ����ʯīϩ��������ΪĿǰ��������֪��������Ӳ�����������ٶ��������Ͳ��ϣ�Ӧ��ǰ��ʮ�ֹ��������й���ʯīϩ��������ȷ����(����)
2010��ŵ��������ѧ�����ڱ����״ΰ��������ʯī�Ŀ�ѧ�ҡ�����ʯī��Ϊʯīϩ�����֡�ֻ��һ��̼ԭ�Ӻ��̼��Ƭ����ʯīϩ��������ΪĿǰ��������֪��������Ӳ�����������ٶ��������Ͳ��ϣ�Ӧ��ǰ��ʮ�ֹ��������й���ʯīϩ��������ȷ����(����)
A��ʯīϩ��̼����
B��ʯīϩ��һ���л���
C��ʯīϩ��̼ԭ�ӵĻ��ϼ�Ϊ��3
D��ʯīϩ�ɵ��磬˵�����ǵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
25 ��ʱ�����и���������ָ����Һ��һ���ܴ����������(����)
A��pH��1����Һ�У�Na����K����MnO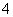 ��CO
��CO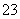
B��c(H��)��1��10��13 mol��L��1����Һ�У�Mg2����Cu2����SO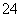 ��NO
��NO
C��0.1 mol��L��1NH4HCO3��Һ�У�K����Na����NO ��Cl��
��Cl��
D��0.1 mol��L��1FeCl3��Һ�У�Fe2����NH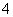 ��SCN����SO
��SCN����SO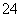
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Na2S2O3����Ҫ�Ļ���ԭ�ϣ�������ˮ�������Ի���Ի������ȶ���
��.�Ʊ�Na2S2O3��5H2O
��Ӧԭ����Na2SO3(aq)��S(s)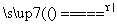 Na2S2O3(aq)
Na2S2O3(aq)
ʵ�鲽�裺
�ٳ�ȡ15 g Na2SO3����Բ����ƿ�У��ټ���80 mL����ˮ����ȡ5 g��ϸ����ۣ���3 mL�Ҵ���ʪ������������Һ�С�
�ڰ�װʵ��װ��(��ͼ��ʾ�����ּг�װ����ȥ)��ˮԡ���ȣ���60 min��
�۳��ȹ��ˣ�����Һˮԡ����Ũ������ȴ����Na2S2O3��5H2O�������ˡ�ϴ�ӡ�����õ���Ʒ��
�ش����⣺
(1)����ڷ�Ӧǰ���Ҵ���ʪ��Ŀ����__________________________��
(2)����a��������________����������____________________��
(3)��Ʒ�г�����δ��Ӧ��Na2SO3�⣬����ܴ��ڵ���������______________�������Ƿ���ڸ����ʵķ�����____________________________��
(4)��ʵ��һ������ڼ��Ի����½��У������Ʒ���ƣ������ӷ�Ӧ����ʽ��ʾ��ԭ��________________________________________________________________________
________________________________________________________________________��
��.�ⶨ��Ʒ����
ȷ��ȡW g��Ʒ������������ˮ�ܽ⣬�Ե�����ָʾ������0.100 0 mol��L��1��ı���Һ�ζ���
��Ӧԭ��Ϊ2S2O ��I2===S4O
��I2===S4O ��2I��
��2I��
(5)�ζ����յ�ʱ����Һ��ɫ�ı仯��____________________________________________��
(6)�ζ���ʼ���յ��Һ��λ����ͼ�������ĵ�ı���Һ���Ϊ__________mL����Ʒ�Ĵ���Ϊ(��Na2S2O3��5H2O��Է�������ΪM)______________��
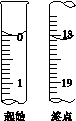
��.Na2S2O3��Ӧ��
(7)Na2S2O3��ԭ�Խ�ǿ������Һ���ױ�Cl2������SO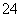 �����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�����������ȼ����÷�Ӧ�����ӷ���ʽΪ____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Һ����һ�����������Σ�Ϊ��ˮ��ϵ�����л������ӡ�Al2Cl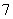 ��AlCl
��AlCl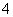 ��ɵ�����Һ�������Һʱ�����ڸ���Ʒ�ϵ������
��ɵ�����Һ�������Һʱ�����ڸ���Ʒ�ϵ������
(1)����Ʒ�ӵ�Դ��________������֪��ƹ����в����������������л������Ӳ�����缫��Ӧ�������缫��ӦʽΪ__________________________________________��������AlCl3ˮ��Һ�����Һ������������Ϊ________��
(2)Ϊ�ⶨ�Ʋ��ȣ���NaOH��Һ�ܽ����Ʒ��������Ʋ㣬����Ӧת��6 mol����ʱ�����û�ԭ��������ʵ���Ϊ________mol��
(3)�����ۺ�Fe2O3�����ȷ�Ӧʵ�飬��Ҫ���Լ�����________��
a��KCl b��KClO3 c��MnO2 d��Mg
ȡ�������ȷ�Ӧ���õĹ������������������ϡH2SO4���μ�KSCN��Һ����������______________(��ܡ��� ���ܡ�)˵��������������Fe2O3��������________(�����ӷ���ʽ˵��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ���ҴӺ����Һ(��H2O�⣬����CCl4��I2��I����)�л��յ⣬��ʵ��������£�
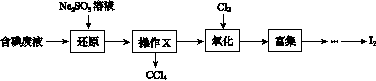
(1)���Һ�м����Թ�����Na2SO3��Һ������Һ�е�I2��ԭΪI���������ӷ���ʽΪ__________________���ò�����I2��ԭΪI����Ŀ����______________________��
(2)����X������Ϊ________��
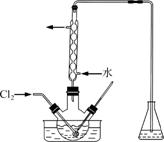
(3)����ʱ����������ƿ�н���I����ˮ��Һ���������pHԼΪ2������ͨ��Cl2����40 �����ҷ�Ӧ(ʵ��װ����ͼ��ʾ)��
ʵ������ڽϵ��¶��½��е�ԭ����______________����ƿ��ʢ�ŵ���ҺΪ________��
(4)��֪��5SO ��2IO
��2IO ��2H��===I2��5SO
��2H��===I2��5SO ��H2O
��H2O
ij�����ˮ(pHԼΪ8)��һ������I2�����ܴ���I����IO �е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO
�е�һ�ֻ����֡��벹���������麬���ˮ���Ƿ���I����IO ��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
��ʵ�鷽����ȡ���������ˮ��CCl4�����ȡ����Һ��ֱ��ˮ���õ�����Һ���鲻���еⵥ�ʴ��ڣ�________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
ʵ���пɹ�ѡ����Լ���ϡ���ᡢ������Һ��FeCl3��Һ��Na2SO3��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ͭ������������Ԫ�أ�Ҳ����������ʹ�õĽ���֮һ��ͭ��������ʹ�öԹ��������������涼��������Զ��Ӱ�졣
(1)д��ͭ��ϡ���ᷴӦ�Ļ�ѧ����ʽ��________________________________________��
(2)Ϊ�˱��������ͽ�Լ��Դ��ͨ������H2O2��ϡ����Ļ����Һ�ܳ��Ͼ�ӡˢ��·���е�ͭ������ʵ��ͭ�Ļ������á�д���ܳ�ͭ�����ӷ���ʽ��__________________________________________��
(3)��ҵ���Ի�ͭ��Ϊԭ�ϣ����û�������������ͭ���ù��յ��м���̻ᷢ����Ӧ��2Cu2O��Cu2S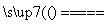 6Cu��SO2���÷�Ӧ�е���������____________��������19.2 g Cuʱ����Ӧ��ת�Ƶĵ��ӵ����ʵ���Ϊ________________________________________________________________________mol��
6Cu��SO2���÷�Ӧ�е���������____________��������19.2 g Cuʱ����Ӧ��ת�Ƶĵ��ӵ����ʵ���Ϊ________________________________________________________________________mol��
(4)ͭ�ڳ�ʪ�Ŀ������ܷ���������ʴ�����⣬ͭ�����Ҫ�ɷ�ΪCu2(OH)2CO3(��ʽ̼��ͭ)����д�����������и����ĵ缫��Ӧʽ��______________________________________________________________��
(5)�о���ѧϰС���á���ӵ��������ⶨij������CuSO4��5H2O(��������I����Ӧ������������)�ĺ�����ȡa g�������100 mL��Һ��ÿ��ȡ25.00 mL����Һ���μ�KI��Һ���а�ɫ�⻯��������ɣ�д���÷�Ӧ�����ӷ���ʽ��____________________________�������μ�KI��Һ���������ٲ�������Һ�е�I2����������Ʊ���Һ�ζ���������Ӧ�Ļ�ѧ����ʽΪI2��2Na2S2O3===2NaI��Na2S4O6��ƽ������c mol/L Na2S2O3��Һ�����ΪV mL����������CuSO4��5H2O����������Ϊ________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ֱ����뷴Ӧ����ʽ��Ӧ����ȷ����(����)
A����������������Fe2O3��6H��===2Fe3����3H2O
B���������ʵ�����KHCO3��Ba(OH)2��Һ��ϣ�
HCO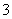 ��Ba2����OH��===BaCO3����H2O
��Ba2����OH��===BaCO3����H2O
C�����Ȼ�����Һ�м��������ˮ��Al3����4NH3��H2O===AlO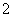 ��4NH
��4NH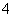 ��2H2O
��2H2O
D������ˮ��Һ�ʼ��ԣ�S2����H2O HS����OH��
HS����OH��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com