
��14�֣��������Ƴ�����Ư����ɱ���������������������Ʊ��治�����ױ��ʡ�
��1��ij����С����̽��һ������������Ʒ�Ƿ��Ѿ����ʣ�
ȡ������Ʒ���ܽ⣬����_______��Һ��������а�ɫ������֤��Na2O2�Ѿ����ʡ�
��2���ÿ���С��Ϊ�˴��Բⶨ�������Ƶ��������������dz�ȡag��Ʒ�����������ͼװ�����ⶨ�������Ƶ�����������

�ٽ��������Ӻ��Ժ�����еĵ�һ��������_______��
��д��װ��C�з�����Ӧ�Ļ�ѧ����ʽ��_______��
��ʵ�����ʱ���ڶ�ȡʵ����������������ʱ������Ϊ��������_______ (��ѡ���ţ���
a��ֱ�Ӷ�ȡ���������������ȴ������
b��������Ͳ����Һ��߶�ʹ֮��ͬ
c�������밼Һ�����͵���ƽ��ȡ��Ͳ��ˮ�����
�ܶ�����Ͳ��ˮ�����������ɱ�״�������������ΪVmL������Ʒ�й������Ƶ���������Ϊ_______��
�ݱ�ʵ�鷽�����ڵ�ȱ����_______��
��14�֣���1��BaCl2��Һ��2�֣��������𰸶����֣�
��2���� ���װ�õ������ԣ�2�֣�
��2CO2+ 2Na2O2= 2Na2CO3+O2 ��3�֣�
��bc(2��)
��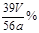 ��3�֣�
��3�֣�
��E��F֮�䵼����ˮ�����û�а취������������2�֣����𰸺��������֣�
��������
�����������1��Na2O2�ױ��ʣ��������CO2��Ӧ������Na2CO3��Na2CO3��BaCl2��Һ��Ba(NO3)2��Һ����Ӧ�����ɰ�ɫBaCO3��������Na2O2��ˮ��Ӧ������Һ������BaCl2��Һ��Ba(NO3)2��Һ��Ӧ����2���ٸ������⣬��ʵ��ɹ��Ĺؼ���ȷ������Ӧ�����������������˱���������װ���Ƿ�©������ͼ��A��B��C��D��E��Fװ�õ�Ŀ�ķֱ�����ȡCO2����ȥCO2�л��е�HCl����ȡ��������ȥO2���е�CO2���ռ���������ȡ�������������C����Ҫ��ӦʽΪ2CO2+2Na2O2=2Na2CO3+O2����a��C�з�Ӧ�Ƿ��ȷ�Ӧ�������������¶ȸ������£��ʲ���ȴ���������������ƫ����b����Ͳ����Һ��߶Ȳ���ͬ��������ѹǿ��������ѹ��ͬ���������������ȷ������ƽ�Ӷ��������������������Ͳ��ˮ���������ȷ������V/Vm��֪n(O2)=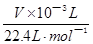 ���ɷ�Ӧʽ2CO2+2Na2O2=2Na2CO3+O2��֪��n(Na2O2)=2n(O2)=2��
���ɷ�Ӧʽ2CO2+2Na2O2=2Na2CO3+O2��֪��n(Na2O2)=2n(O2)=2��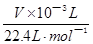 ����n•M��֪��m(O2)=2��
����n•M��֪��m(O2)=2��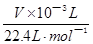 ��78g•mol��1������Ʒ��Na2O2����������=
��78g•mol��1������Ʒ��Na2O2����������=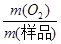 ��100%=
��100%=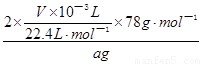 ��100%=
��100%=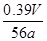 ��100%��
��100%��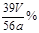 ���ݸ�ʵ��ɹ��Ĺؼ���ȷ������Ӧ���������������E��F֮�䵼����ˮ�����û�а취������������
���ݸ�ʵ��ɹ��Ĺؼ���ȷ������Ӧ���������������E��F֮�䵼����ˮ�����û�а취������������
���㣺����������ƺ�̼���Ƶ���Ҫ���ʡ��ⶨ�������Ƶ�����������CO2���Ʊ���������ռ�������ԭ����������������ʵ��������ڻ�ѧ����ʽ�����е�Ӧ�õ����֪ʶ��


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������Ƴ�����Ư����ɱ���������������������Ʊ��治�����ױ��ʡ�
��1��ij����С����̽��һ������������Ʒ�Ƿ��Ѿ����ʣ�ȡ������Ʒ�ܽ⣬����_______��Һ��������а�ɫ������֤��Na2O2�Ѿ����ʡ�
��2���ÿ���С��Ϊ�˴��Բⶨ�������Ƶ��������������dz�ȡag��Ʒ�����������ͼװ�����ⶨ�������Ƶ�����������
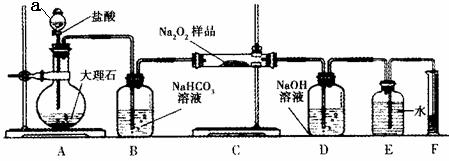
�� װ��������a�������� ��
�� ���������Ӻ��Ժ�����еĵ�һ��������_______��
�� д��װ��C�з�����Ӧ�Ļ�ѧ����ʽ_______��
�� ʵ�����ʱ���ڶ�ȡʵ����������������ʱ������Ϊ��������_______ (��ѡ���ţ���
a��ֱ�Ӷ�ȡ���������������ȴ������
b��������ͲʹE��F��Һ��߶���ͬ
c�������밼Һ�����͵���ƽ��ȡ��Ͳ��ˮ�����
�ݶ�����Ͳ��ˮ�����������ɱ�״�������������ΪVmL������Ʒ�й������Ƶ���������Ϊ_______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com