
| |||||||||||||||||||||||
(1) |
������,Al(OH)3 |
(2) |
���ȼ�����KClO3������Mg���������ȼ |
(3) |
4Fe(OH)2��O2��2H2O��4Fe(OH)3 |
(4) |
Al2O3��2OH����3H2O��2[Al(OH)4]�� |
(5) |
�����𰸣�Al3+��3H2O ����������������һ�����͵�����ͼ�ƶ��⣮���������Ԫ�ػ��������֪ʶ�����գ�����Ҫ����ѧ����֪ʶ����ۺ�������������D��һ�ֺ���ɫ�����֪DΪFe2O3����J��Fe(OH)3��G��Fe(OH)2��F��FeCl2��E��Fe����Ͽ�ͼ��Ϣ����֪�������������ʷֱ�Ϊ��A��Al2O3��B��O2��C��Al��H��Na[Al(OH)4]��I��AlCl3��֪����ÿ�����ʣ����⼴������ |


 �߽�������ϵ�д�
�߽�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ���ݽ�����ѧ2009-2010ѧ��߶���ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�022
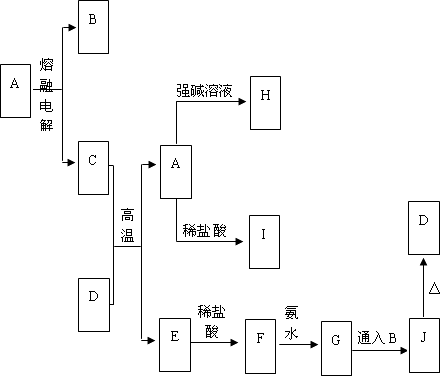
A��J����ѧ��ѧ���������ʣ�����֮���ת����ϵ����ͼ��ʾ(���ֲ����Ѿ���ȥ)����֪A��һ�ָ��۵�Ļ����D��һ�ֺ���ɫ�Ĺ��壬H����ɫ��Ӧ�ʻ�ɫ��
��ش��������⣺
(1)A���ʵĻ�ѧʽΪ________��H���ʵĻ�ѧʽΪ________��
(2)G��J�Ļ�ѧ����ʽΪ________________��
(3)��D������ǡ�÷�Ӧ���ķ�Ӧ�����ӷ���ʽΪ________________��������������Һ��pH________(����ڡ�С�ڡ�����)7����ԭ����(�����ӷ���ʽ��ʾ)________________��
(4)������E�ڳ�ʪ�Ŀ����������绯ѧ��ʴ���为�������ĵ缫��Ӧʽ��________________�����������ĵ缫��Ӧʽ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��������ѧ��2007��Ӧ���ڶ����¿��Ծ�����ѧ���⣨�˽̰棩 �˽̰� ���ͣ�022
| |||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
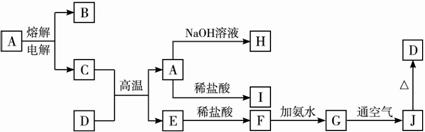
��ش��������⣺?
��1��A���ʵĻ�ѧʽΪ_________________��H���ʵ�����Ϊ____________________��?
��2��GJ�Ļ�ѧ����ʽΪ_____________________��
��3��D����������ǡ�÷�Ӧ�����ӷ���ʽΪ__________________��������Һ�����Ե�ԭ����__________________�������ӷ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com