
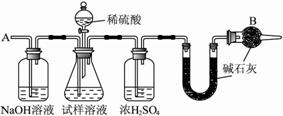
��Ҫʵ�鲽�����£�?
�ٰ�ͼ��װ������������װ�õ�������?
�ڽ�a g����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ?
�۳���ʢ�м�ʯ�ҵ�U�ιܵ��������õ�b g?
�ܴӷ�Һ©������6 mol��L��1�����ᣬֱ�����ٲ�������ʱΪֹ?
�ݴӵ���A����������һ�����Ŀ���?
���ٴγ���ʢ�м�ʯ�ҵ�U�ιܵ��������õ�c g?
���ظ�����ݺ͢IJ�����ֱ��U�ιܵ������������䣬Ϊd g?
����պ�������⣺?
��1������������ƽ������Ʒʱ�������ƽ��ָ������ƫת��˵��__________��?
��2��װ���и����B��������_______________________��?
��3���������Һ©���е����ỻ��Ũ����ͬ�����ᣬ���ԵĽ��____________________���ƫ�ߡ���ƫ�͡����䡱����?
��4������ݵ�Ŀ����______________________��?
��5������ߵ�Ŀ����_________________________��?
��6�������д�������������ļ���ʽΪ_____________��?
��7��������������ʵ�鷽���ⶨ�����д�������������������һ�ֲ�ͬ��ʵ�鷽����???
���������⿼�鶨��ʵ�����⡢��������ʵ�鷽����Ƶȣ�����һ���Ŀ����ԡ�������Ŀ�����������������ͨ��U�ι������ı仯�����ģ����Ը����B�������Ƿ�ֹ�����е�ˮ������CO2����U�ι��С����һ�ʵ�ʵ���漰���Դ����¼���������п��ǣ���̼������ӵĽǶȷ��������������м������������Ը��λ��εȵȣ��������ӽǶȿ��ǣ�����������Ʒ�м������������ᣬȻ���������м�����������������Һ�ȵȡ�?
�𰸣���1����Ʒ�أ�������?
��2����ֹ�����е�CO2��ˮ������U�ι���?
��3��ƫ��?
��4���ѷ�Ӧ�е�CO2ȫ������U�ι���?
��5���жϷ�Ӧ�е�CO2�Ƿ�ȫ���ų�������U�ι��еļ�ʯ������?
��6��![]() ��100%?
��100%?
��7��ȡһ�����Ĵ�����������������������ˮ�У�����������BaCl2��Һ�����ˡ�ϴ�ӡ��������������BaCO3���������������𰸣���?



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 106(d-b) |
| 44a |
| 106(d-b) |
| 44a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
- 3 |
- 3 |
| ||
| 106(d-b) |
| 44a |
| 106(d-b) |
| 44a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 106(d-b) |
| 44 |
| 106(d-b) |
| 44 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 106(d-b) |
| 44a |
| 106(d-b) |
| 44a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����������������ѧ��һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ�ʵ����
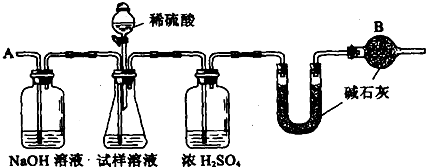
(10��).��֪ij���������к��������Ȼ��ƣ�Ϊ�ⶨ�����д��������������������ͼװ�ý���ʵ�顣

��Ҫ�������£�����գ�
�� ��ͼ��װ�����������װ�õ������Ԣ� ��a g����������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ���� ����ʢ�м�ʯ�ҵ�U�ιܵ�����Ϊb g���� �ӷ�Һ©���е���6mol/L��ϡ���ᣬֱ�����ٲ�������Ϊֹ���� �ӵ���A����������һ�����Ŀ������� �ٴγ���ʢ�м�ʯ�ҵ�U�ιܵ�����Ϊc g���� �ظ��ݺ͢IJ�����ֱ��U�ιܵ������������䣬�������Ϊd g��
�ش��������⣺(��ʯ����һ�ָ����,���ܸ�����������)
(1)װ���и����B��������
(2)�������Һ©���е����ỻ��ͬŨ�ȵ�����,���ԵĽ���� (��ƫ��.ƫ�ͻ�)
(3)����ݵ�Ŀ����
(4)����ߵ�Ŀ����
(5)�������д�������������ļ���ʽΪ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com