
ϩ�����廯�⡢ˮ�ӳ�ʱ������������֮�֣����磺
CH2��CHCH3��HBr��CH3CHBrCH3(��Ҫ����)��CH3CH2CH2Br(��Ҫ����)
CH2��CHCH2CH3��H2O![]() CH3CH(OH)CH2CH3(��Ҫ����)��CH3CH2CH2CH2OH(��Ҫ����)
CH3CH(OH)CH2CH3(��Ҫ����)��CH3CH2CH2CH2OH(��Ҫ����)
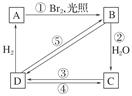
����ͼ��B��C��D������ط�Ӧ�е���Ҫ����(���ַ�Ӧ�������Լ���ʡ��)���һ�����B�н���4��̼ԭ�ӡ�1����ԭ�ӡ�9����ԭ�ӣ���ͼ����B�Ľṹ��ʽΪ________������ȡ����Ӧ����________(���ͼ�е���ţ���ͬ)��������ȥ��Ӧ����________��д����Ӧ�۵Ļ�ѧ����ʽ(ֻд��Ҫ�����д��Ӧ����)________��

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ϩ����� | ������� |
(CH3)2C=CHCH3 | 10.4 |
CH3CH=CH2 | 2.03 |
CH2=CH2 | 1.00 |
CH2=CHBr | 0.04 |
�ݱ������ݣ��ܽ�ϩ���������ӳ�ʱ����Ӧ������C=C��ȡ���������ࡢ������Ĺ�ϵ��_________________________________��
(2)���л��������Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ���������������ƣ����з�Ӧ������������_________(�����)��
A.(CH3)2C=CHCH3 B.CH3CH=CH2
C.CH2=CH2 D.CH2=CHBr
(3)ϩ�����廯�⡢ˮ�ӳ�ʱ������������֮�֣����磺

���п�ͼ��B��C��D������ط�Ӧ�е���Ҫ����(�����������Լ���ʡ��)���һ�����B�н���4��̼ԭ�ӡ�1����ԭ�ӡ�1����ԭ�ӡ�
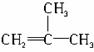
������ͼ�У�B�Ľṹ��ʽΪ________________������ȡ����Ӧ����____________(���ͼ�����)��������ȥ��Ӧ����___________(�����)��д����Ӧ�ܵĻ�ѧ����ʽ(ֻд��Ҫ���������Ӧ����)____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ϩ����� | ������� |
��CH3��2C=CHCH3 | 10.4 |
CH3CH=CH2 | 2.03 |
CH2=CH2 | 1.00 |
CH2=CHBr | 0.04 |
(1)���л��������Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ������й������ƣ����з�Ӧ����������_______________������ţ���
A.��CH3��2C=C��CH3��2
B.CH3CHCH=CH2CH3
C.CH2=CHCH3
D.CH2=CHCl
��2��ϩ�����廯�⡢ˮ�ӳ�ʱ������������֮�֣����磺CH2=CHCH3+HBr![]() CH3CHBrCH3����Ҫ���+CH3CH2CH2Br����Ҫ�����CH2=CHCH2CH3+H2O
CH3CHBrCH3����Ҫ���+CH3CH2CH2Br����Ҫ�����CH2=CHCH2CH3+H2O![]() CH3CH��OH��CH2CH3����Ҫ���+CH3CH2CH2CH2OH����Ҫ���
CH3CH��OH��CH2CH3����Ҫ���+CH3CH2CH2CH2OH����Ҫ���
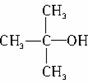
��д��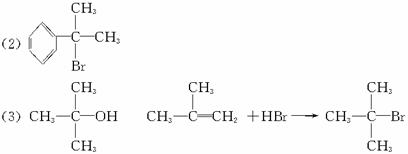 ��HBr��Ӧ����Ҫ����Ľṹ��ʽ____________________��
��HBr��Ӧ����Ҫ����Ľṹ��ʽ____________________��
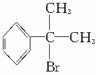
��3�����п�ͼ��B��C��D������ط�Ӧ�е���Ҫ��������������Լ���ʡ�ԣ����һ�����B�н����ĸ�̼ԭ�ӡ�һ����ԭ�ӡ�һ����ԭ�ӡ���ͼ�У�C�Ľṹ��ʽΪ____________��д����Ӧ�ݵĻ�ѧ����ʽ��ֻд����Ҫ���________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��20�֣�
��1���±�Ϊϩ��������巢���ӳɷ�Ӧ��������ʣ�����ϩΪ������
| ϩ����� | ������� |
| (CH3)2C��CHCH3 | 10��4 |
| CH3CH��CH2 | 2��03 |
| CH2��CH2 | 1��00 |
| CH2��CHBr | 0��04 |
�ݱ������ݣ��ܽ�ϩ��������ʱ����Ӧ������C��C��ȡ���������ࡢ������Ĺ�ϵ��
_____________________________________________________��
��2�����л��������Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ���������������ƣ����з�Ӧ������������______________������ţ���
A��(CH3)2C��C(CH3) 2 B��CH3CH��CH CH3
C��CH2��CH2 D��CH2��CHCl
��3��ϩ�����廯�⡢ˮ�ӳ�ʱ������������֮�֣����磺
���п�ͼ��B��C��D������ط�Ӧ�е���Ҫ��������������Լ���ʡ�ԣ����һ�����B�н���4��̼ԭ�ӡ�1����ԭ�ӡ�1����ԭ�ӡ�
��������ͼ�У�B�Ľṹ��ʽΪ____________________________��
������ȡ����Ӧ����________�����ͼ����ţ���
������ȥ��Ӧ����__________������ţ�
��д����Ӧ�ܵĻ�ѧ����ʽ��ֻд��Ҫ���������Ӧ��������
_______ ____________________________��
��д����Ӧ�Ļ�ѧ����ʽ��_______ ______________________��
�� A�� B��C��D����һ�������ܷ����ۺϷ�Ӧ��д���þۺϷ�Ӧ�ķ���ʽ
______________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��߿���ѧһ�ָ�ϰ����ʶ�л���Ľṹ����ࡢ����������ר���ۺϲ��� ���ͣ������
(8��)(1)����Ϊϩ��������巢���ӳɷ�Ӧ���������(����ϩΪ��)��
| ϩ����� | ������� |
| (CH3)2C===CHCH3 | 10.4 |
| CH3CH===CH2 | 2.03 |
| CH2===CH2 | 1.00 |
| CH2===CHBr | 0.04 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��ӱ�������ѧ�߶���ѧ�ڵ�һ�ο��Ի�ѧ�Ծ� ���ͣ������
(8��)�±�Ϊϩ��������巢���ӳɷ�Ӧ��������ʣ�����ϩΪ������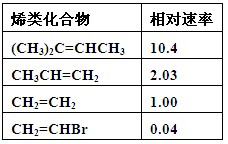
��1�����л��������Ȼ���ӳ�ʱ��ȡ���������ʵ�Ӱ������й������ƣ����з�Ӧ����������_______________������ţ���
A����CH3��2C=C(CH3)2 B��CH3CH=CHCH2CH3
C��CH2="CH" CH3 D��CH2=CHCl
��2��ϩ�����廯�⡢ˮ�ӳ�ʱ������������֮�֣����磺
CH2=CHCH3 + HBr �� CH3CHBrCH3 + CH3CH2CH2Br
����Ҫ��� ����Ҫ���
CH2=CHCH2CH3 + H2O  CH3CH(OH)CH2CH3 + CH3CH2CH2CH2OH
CH3CH(OH)CH2CH3 + CH3CH2CH2CH2OH
����Ҫ��� ����Ҫ���
a. ��д�� ��HBr��Ӧ����Ҫ����Ľṹ��ʽ___________________��
��HBr��Ӧ����Ҫ����Ľṹ��ʽ___________________��
b. ���п�ͼ��B��C��D������ط�Ӧ�е���Ҫ��������������Լ���ʡ�ԣ����һ�����B�н����ĸ�̼ԭ�ӡ�һ����ԭ�ӡ�һ����ԭ�ӡ���ͼ�У�C�Ľṹ��ʽΪ________________________��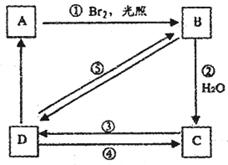
д����Ӧ�ݵĻ�ѧ����ʽ��ֻд��Ҫ���������Ӧ��������__________________________________ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com