���
�⣺��1��2 I
2��s��+5O
2��g��=2 I
2O
5��s������H=-75.56kJ?mol
-1�٣�
2CO��g��+O
2��g��=2 CO
2��g������H=-566.0kJ?mol
-1�ڣ�
������ʽ�ڡ�
-�١�
��5CO��g��+I
2O
5��s��=5 CO
2��g��+I
2��s������H=��-566.0kJ?mol
-1����
-��-75.56kJ?mol
-1����
=-1377.22kJ/mol��
�������Ȼ�ѧ��Ӧ����ʽΪ��5CO��g��+I
2O
5��s��=5 CO
2��g��+I
2��s������H=-1377.22kJ/mol��
�ʴ�Ϊ��5CO��g��+I
2O
5��s��=5 CO
2��g��+I
2��s������H=-1377.22kJ/mol��
��2������Ӧ�Ƿ�Ӧǰ���������������ķ�Ӧ������ϵ��ѹǿ���ֲ��䣬��a����˵����Ӧ�Ѵﵽƽ��״̬�����ŷ�Ӧ�Ľ��У�NO
2��Ũ�ȼ�С����ɫ��dz����b����˵����Ӧ�Ѵ�ƽ�⣻SO
3��NO�����������������1��1����c������Ϊƽ��״̬���ж����ݣ�d���������������ʶ����淴Ӧ���ʣ�������Ϊƽ��״̬���ж����ݣ���ѡb��
NO
2��g��+SO
2��g��?SO
3��g��+NO��g��
��ʼ���ʵ���� 1a 2a 0 0
ת�����ʵ���� x x x x
ƽ�����ʵ���� 1a-x 2a-x x x
��1a-x������2a-x��=1��6����x=
a����ƽ�ⳣ��Ϊ=
=
��
�ʴ�Ϊ��b��2.67��
��
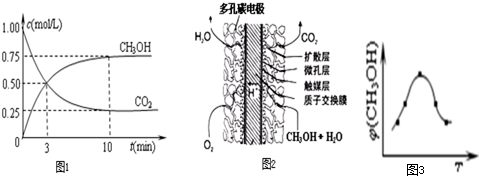
��3��������ͼ��֪��ߵ㷴Ӧ����ƽ�⣬��ƽ����¶�Խ�ߣ��գ�CH
3OH��ԽС��ƽ�����淴Ӧ���У������¶�ƽ�����ȷ�����У��淴ӦΪ���ȷ�Ӧ��������ӦΪ���ȷ�Ӧ������H��0���ʴ�Ϊ������
�����ݻ�Ϊ1L�ĺ����ܱ������г���1mol CO
2��3mol H
2������������Ӧ�����CO
2��CH
3OH��g����Ũ����ʱ��仯��ͼ1��ʾ��ƽ��Ũ��c��CO
2��=0.25mol/L��c��CH
3OH��=0.75mol/L����
CO
2 ��g��+3H
2��g��?CH
3OH��g��+H
2O��g��
��ʼ����mol/L�� 1 3 0 0
�仯����mol/L�� 0.75 2.25 0.75 0.75
ƽ������mol/L�� 0.25 0.75 0.75 0.75
ƽ�ⳣ��K=
=5.3
��������ƽ����ϵ���ٳ�0.5mol CO
2��1.5mol ˮ�����������¶Ȳ��䣩��Ũ����Q=
| 0.75��(0.75+1.5) |
| (0.25+0.25)��0.753 |
=5.3=K����ƽ�ⲻ�ƶ���
�ʴ�Ϊ������
��ֱ�Ӽ״�ȼ�ϵ�ؽṹ��ͼ2��ʾ��ͼʾ�����жϣ��������ҺΪ������Һ���״�ȼ���ڸ���ʧ���ӷ���������Ӧ���ɶ�����̼����ϵ���غ���д�����缫��ӦΪ��CH
3OH-6e
-+H
2O=6H
++CO
2��
�ʴ�Ϊ��CH
3OH-6e
-+H
2O=6H
++CO
2��




 ������������Ϥ�ļ������壬���п�����ͼ��ʾװ�ø��ﲢ�ռ����ǣ�ʡ��������̨����������H2 ��O2 ��CO2 ��SO2 ��CH4
������������Ϥ�ļ������壬���п�����ͼ��ʾװ�ø��ﲢ�ռ����ǣ�ʡ��������̨����������H2 ��O2 ��CO2 ��SO2 ��CH4

 ���ģ�ͣ�
���ģ�ͣ�