
���� ��1��A�������е�̼�⻯�����NOx��Ϊһ����Ⱦ���̫�������������������ܷ�����ѧ��Ӧ���������ֶ�����Ⱦ���һ����Ⱦ��Ͷ�����Ⱦ��Ļ�������Ϳ�������γɵ�������Ⱦ����Ϊ�⻯ѧ������
B��������ָpH��5.6�Ľ�ˮ��
C��PM2.5���γ���������������ף�
D��������̼�ᵼ������ЧӦ��
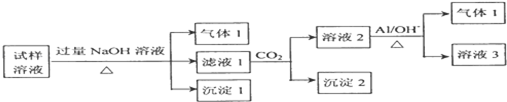
��2��������Һ�м������NaOH�����ȣ����ɵ�����1��������1������NH3���������к���NH4+�����ɵij���1ΪMg��OH��2��˵��ԭ��Һ�к�Mg2+������Һ��ͨ��CO2���õ���Һ2������2����Һ2�м���Al��NO3-+A1+OH-+H2O��NH3��+[Al��OH��4]-����������2����������NH3��������֪����֪����Һ2�к���NO3-������Ԫ���غ�֪��ԭ��Һ�к���NO3-����Һ1��ͨ��CO2���õ�����2�������2ΪAl��OH��3��˵��ԭ��Һ�к�Al3+���ݴ˷�����
��3��������п����������ɫ��ζ�������壬˵�������������������Һ�����ԣ���CO32-��NO3-���ܴ������棻����NaOH��Һ��������ɫ������˵�������������ӣ����ݲ����ij����������NaOH�����ʵ���֮��Ĺ�ϵͼ��֪����Һ��һ������Mg2+��Al3+������Ϊ�������ﵽ���ֵʱ�����������������ƣ��������䣬��˵����Һ�л�����NH4+��������Һ�л�������������ӣ�����һ������SO42-�����ͼ���и������ĵ��������ƣ�������Һ��n��H+����n��Mg2+����n��Al3+����n��NH4+�����ݴ˽��
��� �⣺��1��A���⻯ѧ��������������������ȾԴ���������̼�⻯���HC���͵������NOx����һ����Ⱦ�������⣨����⣩�����»ᷢ���⻯ѧ��Ӧ���ɶ�����Ⱦ���A��ȷ��
B�����ڿ����ж�����̼�����룬�����Ľ����pHҲС��7����������ָpH��5.6�Ľ�ˮ����B����
C��PM2.5���γ���������������ף��ǵ�����������Ҫԭ��֮һ����C��ȷ��
D��������̼�ᵼ������ЧӦ�����γ�����ЧӦ����Ҫԭ��������ն�����D��ȷ��
��ѡAC��
��2��������Һ�м������NaOH�����ȣ����ɵ�����1��������1ֻ����NH3���������к���NH4+�������������ƹ����������ɵij���1ΪMg��OH��2��˵��ԭ��Һ�к�Mg2+�����ԭ��Һ�к�Al3+������������������Ʒ�Ӧת��Ϊ��AlO2-������Һ1�п��ܺ�AlO2-��SO42-��NO3-��Cl-��һ����Na+��OH-��������Һ1��ͨ��CO2���õ���Һ2����Һ2�м���Al����������2��������Ϣ�����ķ�ӦNO3-+A1+OH-+H2O��NH3��+[Al��OH��4]-����������NH3��������֪����֪����Һ2�к���NO3-������Ԫ���غ�֪��ԭ��Һ�к���NO3-�����õ�����2����˵����Һ1�к�AlO2-����ԭ��Һ�к�Al3+��
����Һ��һ�����ڵ�������NH4+��Mg2+��Al3+��NO3-��Na+��SO42-��Cl-�Ĵ��ڲ���ȷ����
��������Һ�������NaOH��Һ�����ɳ�����ΪMg��OH��2����������������Һ�е�笠���������������Ӧ���ɵģ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O�����ڰ�����Ψһ�ļ������壬�ʼ���ķ���Ϊ����ʪ��ĺ�ɫʯ����ֽճ�ڲ������ϣ���������ƿ������ֽ��������˵��������Ϊ�������ʴ�Ϊ��
Mg��OH��2��NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O����ʪ��ĺ�ɫʯ����ֽճ�ڲ������ϣ���������ƿ������ֽ��������˵��������Ϊ������
�ھ������������������й�һ�����е�����ΪNH4+��Mg2+��Al3+��NO3-�����ڲ���ȷ������������SO42-��Cl-��������SO42-�ñ��Σ�����Cl-����������������Ag+������SO42-������Cl-��Ӧ����Ba2+ֻ��SO42-��Ӧ����Ӧ������Һ�м���Ba��NO3��2�����а�ɫ�������ɣ���˵����Һ����SO42-��Ȼ��ȡ�ϲ���Һ������������������а�ɫ�������ɣ�����Һ�к�Cl-��
�ʴ�Ϊ��NH4+��Mg2+��Al3+��NO3-��Ba��NO3��2�� AgNO3��
��3��������п����������ɫ��ζ�������壬˵�������������������Һ�����ԣ���CO32-��NO3-���ܴ������棻
����NaOH��Һ��������ɫ������˵��������Fe3+�����ݲ����ij����������NaOH�����ʵ���֮��Ĺ�ϵͼ��֪����Һ��һ������Mg2+��Al3+������Ϊ�������ﵽ���ֵʱ�����������������ƣ��������䣬��˵����Һ�л�����NH4+��������Һ�л�������������ӣ�����һ������SO42-��
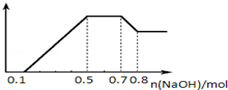
��ͼ���֪����һ��Ϊ���������������Ʒ�Ӧ��������������Ϊ0.1mol����n��H+��=0.1mol��
������Ϊ笠��������������Ʒ�Ӧ��������������Ϊ0.7mol-0.5mol=0.2mol����n��NH4+��=0.2mol��
����Ϊ���������ܽ�����������������������0.8mol-0.7mol=0.1mol����n[Al��OH��3]=0.1mol��������Ԫ���غ��֪n��Al3+��=0.1mol��
�ڶ���Ϊ�������Ƴ���þ���ӡ������ӣ���������������Ϊ0.5mol-0.1mol=0.4mol����n��Mg2+��=��0.4mol-0.1mol��3����2=0.05mol��
A��������������֪����Һ��һ������CO32-��NO3-��һ������SO42-����A����
B��n��Mg2+��=��0.4mol-0.1mol��3����2=0.05mol����B����
C����Һ�е�������Ϊ����������ݵ���غ㣬���ʵ���Ϊ0.35mol����C��ȷ��
D��������������֪����Һ��n��H+����n��Al3+����n��Mg2+��=0.1mol��0.1mol��0.05mol=2��2��1����D����
�ʴ�Ϊ��ABD��
���� ���⿼�������ʵ��ƶϣ���ȷ���ʵ����ʼ����ⷴӦ�����ǽⱾ��ؼ����������ʵ��ܽ��ԡ����ʵ����ʼ������Ϣ�����������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH3 | B�� | CH2=CH2 | C�� | CH3CH=CHCH3 | D�� | CH3CH=CH2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ҵ� | B�� | �ȼ��� | C�� | ������ | D�� | �������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ħ��������g/mol | B�� | ����Ħ�������mol/L | ||
| C�� | �ܽ�ȣ�g/100gH2O | D�� | �ܶȣ�g/cm3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | ||
| C�� |  | D�� |  |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com