
����Ŀ��ԭ������С��36��X��Y��Z��R��W����Ԫ�أ�����X�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Y���γɻ�������������Ԫ�أ�Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�R����ռ���������![]() ��W��ԭ������Ϊ29���ش���������:
��W��ԭ������Ϊ29���ش���������:
��1��Y2X4������Yԭ�ӹ�����ӻ�����Ϊ________��1mol Z2X4���ЦҼ�����ĿΪ ________��
��2��������ZX3�뻯����X2R��VSEPR������ͬ�������幹�Ͳ�ͬ��ZX3�����幹��Ϊ ________�����ֻ���������л�ѧ���ļ��ǽ�С����________���÷���ʽ��ʾ����ͬ)��
��3����Rͬ��������ַǽ���Ԫ����X���γɽṹ���Ƶ��������ʣ����Ʋ����ߵ��ȶ����ɴ�С��˳��________�������� ________�����ߵķе��ɸߵ��͵�˳���� ________������ԭ��________��
��4��Ԫ��Y��һ����������Ԫ��Z�ĵ��ʻ�Ϊ�ȵ����壬Ԫ��Y������������ķ���ʽ��________��
��5��WԪ����________���˶�״̬��ͬ�ĵ��ӣ����̬ԭ�ӵļ۵����Ų�ʽΪ________��
���𰸡�sp2 5NA ������ H2O H2O >H2S> H2Se �뾶Se>S>O������H-Se> H-S> H-O������Խ�̣�����Խ����Խ�ȶ� H2O > H2Se>H2S H2O�γɷ��Ӽ������H2Se��Է�����������H2S�����Ӽ�������Խ���۷е�Խ�� CO 29 3d104s1
��������
ԭ������С��36��X��Y��Z��R��W����Ԫ�أ�����X�����ڱ��а뾶��С��Ԫ�أ���X��HԪ�أ�Y���γɻ�������������Ԫ�أ���Y��CԪ�أ�Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ���Z��NԪ�أ�R����ռ���������1/5��R��O��W��ԭ������Ϊ29����W��CuԪ�أ��ٽ�����ʽṹ�������
��1��C2H4����ƽ���νṹ������Cԭ�ӹ�����ӻ�����Ϊsp2�ӻ����������ǦҼ�����һ��N2H4�����к���5���Ҽ���
�ʴ�Ϊ��sp2 ��5NA��
��2��������NH3�뻯����H2O��VSEPR������ʣ����������壬�����幹�Ͳ�ͬ�����������к���1�Թ¶Ե��ӣ������幹��Ϊ�����Σ�ˮ�����к���2�Թ¶Ե��ӣ��������ֻ���������л�ѧ���ļ��ǽ�С����H2O��
�ʴ�Ϊ�������Σ�H2O��
��3������ͬ������ϵ���ԭ�Ӱ뾶����������Խ�̣�����Խ������Խ�ȶ��������ȶ�����H2O��H2S��H2Se������H2O�γɷ��Ӽ������H2Se��Է�����������H2S�����Ӽ�������Խ���۷е�Խ�ߣ���е��С˳����H2O > H2Se>H2S��
�ʴ�Ϊ��H2O >H2S> H2Se���뾶Se>S>O������H-Se> H-S> H-O������Խ�̣�����Խ����Խ�ȶ���H2O > H2Se>H2S��H2O�γɷ��Ӽ������H2Se��Է�����������H2S�����Ӽ�������Խ���۷е�Խ�ߣ�
��4��Ԫ��C��һ����������Ԫ��N�ĵ��ʻ�Ϊ�ȵ����壬CO��N2��Ϊ�ȵ����壬����Ԫ��Y������������ķ���ʽ��CO��
�ʴ�Ϊ��CO��
��5��ͭԪ��ԭ��������29��������29���˶�״̬��ͬ�ĵ��ӣ����̬ԭ�ӵļ۵����Ų�ʽΪ3d104s1��
�ʴ�Ϊ��29 ��3d104s1��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����̼����(Na2CO4)��һ�ֺܺõĹ�����������ϡ���ᷴӦ�Ļ�ѧ����ʽΪ2Na2CO4��4HCl=4NaCl��2CO2����O2����2H2O�����۹�̼����һ�㶼����̼���ƣ�Ϊ�ⶨij��̼������Ʒ(ֻ��Na2CO4��Na2CO3)�Ĵ��ȣ�ij��ѧ��ȤС������������ַ���ʵʩ��
����һ��![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
![]()
(1)�����ٺ͢۵����Ʒֱ�Ϊ______________________��
(2)���������У�ʹ�õ����������� _________________(��������)��
(3)����������۵IJ������̣�________________________________________________��
������������ͼ��ʾ��װ��ʵ��װ�ã�QΪһ��������������ȡ������Ʒ�����У���Һ©����������ϡ�����������������ַ�Ӧ��

(4)Ϊ�ⶨ��Ӧ������������������ϡ����ǰ����ر�______(����K1����K2������K3������ͬ)����________������A��������_____________________________________________��
(5)��������Ӧֹͣ��ʹK1��K3���ڹر�״̬��K2���ڴ�״̬���ٻ�����Kl�� B��װ�Ĺ����Լ���________________________��
(6)ʵ�����ʱ����Ͳ������x mLˮ����Ͳ�����ռ�����y mL���壬����Ʒ�й�̼���Ƶ�����������___________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij����﮿������ε�طŵ������ͼ��ʾ�����ڸõ�ص�������ȷ����
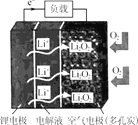
A.���ҺӦѡ��ɴ���Li����ˮ��Һ
B.���ʱ��Ӧ��﮵缫���Դ��������
C.�ŵ�ʱ�������缫�Ϸ����ĵ缫��ӦΪ 2Li����O2��2e��=Li2O2
D.���ʱ������·��ת��0.5mol���ӣ������缫������������3.5g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڱ�״���½��мס��ҡ�������ʵ�飺��ȡ200mLͬŨ�ȵ����ᣬ���벻ͬ������ͬһ��þ���Ͻ��ĩ���������壬�й����ݼ�¼���£�
ʵ����� | �� | �� | �� |
�Ͻ�����/g | 2.55 | 3.85 | 4.59 |
�����������/L | 2.80 | 3.36 | 3.36 |
�Իش�
(1)��������ʵ���Ũ��Ϊ________����������λ��Ч���֣�
(2)�Ͻ���þ�������ʵ���֮��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ�ǵ�3����11~17��Ԫ��ijЩ���ʱ仯���Ƶ�����ͼ�������й�˵������ȷ����( )

A. y���ʾ�Ŀ����ǵ�һ������
B. y���ʾ�Ŀ����ǵ縺��
C. y���ʾ�Ŀ�����ԭ�Ӱ뾶
D. y���ʾ�Ŀ�����ԭ���γɼ�����ת�Ƶĵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1����Ӧ3Fe��S��+4H2O��g��=Fe3O4��s��+4H2��g����һ�ɱ���ݻ����ܱ������н��У��Իش�
������Fe������������Ӧ���ʵı仯��_______���������䡢��С��������ͬ��
�ڽ������������Сһ�룬������Ӧ����________
�۱���������䣬����N2ʹ��ϵѹǿ����������Ӧ����________��
�ܱ���ѹǿ���䣬����N2ʹ�������������������Ӧ����_______��
��2���������ʵ�����A��B�������2 L���ܱ������У��������·�Ӧ��
3A(g)��B(g) xC(g)��2D(g),5 min����c(D)��0.5 mol��L��1��c(A)��c(B)��3��5��C�ķ�Ӧ������0.1 mol��L��1��min��1��
��A��5 minĩ��Ũ����___________���� v(B) ��___________�� �� x��_______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��I.�ڿ�����й©�Ķ������ᱻ�������γ������������������ܽ�����Ⱦ��������ҵ�ϳ���Na2SO3��Һ���ա�����̿��ԭ�ȷ����������������Լ�С�Կ�������Ⱦ��
��1��д����Na2SO3��Һ����SO2�����ӷ���ʽ��________________
��2����ԭ�Ӻ�����________��������ͬ�ĵ��ӡ�д����ԭ���������ӵĹ����ʾʽ��____________
��3��H2O��H2S�ȶ������÷��ӽṹ��֪ʶ���������ɡ�______________________
II.һ���¶��£��̶��ݻ����ܱ������з������з�Ӧ��2C(s)��2SO2(g) ![]() S2(g)��2CO2(g)����Ӧ�����У�������Ũ����ʱ��Ĺ�ϵ��ͼ��
S2(g)��2CO2(g)����Ӧ�����У�������Ũ����ʱ��Ĺ�ϵ��ͼ��
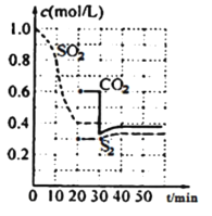
��4���÷�Ӧƽ�ⳣ������ʽΪK=________
��5��0��20min��ѧ��Ӧ���ʱ�ʾv(SO2)=________������ƽ������У����������ܶ�________��ѡ���������С�����䡱��������ԭ��___________________________
��6��30minʱ�ı����������ʹv(��)________v(��)��ѡ����ڡ�����С�ڡ����ڡ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ҹ����гɹ����漰�����У���Ҫ�ɷ�Ϊͬ����Ԫ���γɵ����ǽ������ϵ���
|
|
|
|
A��4.03�״�ھ�̼���跴�侵 | B��2022�궬�»�۰����ٻ��� | C�������ε�Ų���̼������������ | D�������ö������ѺϽ�ɸ���� |
A. AB. BC. CD. D
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������������ѧ�Ŀ�ѧ�һ���˼��������о������N4���ӣ�N4���ӽṹ��ͼ��ʾ�������й�N4��˵����ȷ���ǣ� ��
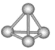
A. N4�ķе��N2��
B. N4������ֻ���зǼ��Լ����Ҽ��ļн�Ϊ109��28��
C. N4������ÿ����ԭ�Ӷ�����8������
D. 1mol N4�к���9 mol���ۼ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com