
��ѧ���о��ϼ���о�������֣���ҵ����ǰ��65����䣬����������е����������ȶ���CO2����һֱ����180 ppm��289 ppm֮�䣬CH4������320 ppb��720 ppb֮�䣬���ڴ����е�CO2��CH4�����ֱ�ߴ�380 ppm��1 700 ppb�������������庬���仯����Ҫԭ���� (����)��
����úΪ��Դ�Ļ����Ĺ㷺ʹ�á�����ʯ��Ϊ��Դ�Ļ����Ĺ㷺ʹ�á�����ԭ����Ϊ��Դ�ĺ˹�ҵ�ķ�չ�����Է��ܡ�̫����Ϊ��Դ���¹�ҵ�ķ�չ
A���٢� B���٢�
C���ڢ� D���ڢ�
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±�Ϊijһ�л���������Լ��ķ�Ӧ�����������л�������� (����)��
| �Լ� | �� | ��ˮ | NaHCO3��Һ |
| ���� | �ų����� | ��ɫ | �ų����� |
A.CH2==CH��CH2��OH
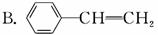
C��CH2==CH��COOH
D��CH3COOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֬������֬�����ܳƣ����Ƕ��ָ�֬����ĸ���������֬������Ҫʳ�������Ҫ�Ļ���ԭ�ϡ���֬���������ʺ���;���京�еIJ�����˫��( )�йص���(����)
)�йص���(����)
A���·��ϵ���֬��������ϴȥ
B��������֬�ڼ��������µ�ˮ�⣬�����������ͺͷ���
C��ֲ����ͨ���⻯��������ֲ������(��������)
D��֬�����л�����֯�ﴢ����������Ҫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B�����л������ʽ����C9H11O2N��
(1)������A����Ȼ�����ʵ�ˮ�������ײⶨ��ʾ�����ӽṹ�в����ڡ�CH3��������A�Ľṹ��ʽΪ
________________________________________________________________________��
(2)������B��ij�ַ���ʽΪC9H12�ķ�����һ�������Ψһ����(�������ڱ�����)��������B�Ľṹ��ʽΪ
________________________________________________________________________��
(3)������A���γɺ�����Ԫ��������C����C�Ľṹ��ʽΪ
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ϊ���������Գ��д�������Ⱦ���ҹ��ɹ��ؿ���������ȼ������Դ�ġ���ɫ�����������������ɱ����ж����л�Ǧ�����������к����ʵ��ŷţ��������������֡���ɫ��������ȼ���� (����)��
A���״� B������
C������ D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����þ������õķ�����ȡ��������,Ȼ��(����)
A.Ͷ���ˮ��
B.Ͷ����ˮ��
C.Ͷ������
D.Ͷ��NaOH��Һ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
)ijͬѧ�о������仯���������ʱ�������������ʵ�鷽����
������:2.7 g Al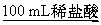 X��Һ
X��Һ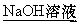 Al(OH)3����
Al(OH)3����
������:2.7 g Al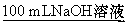 Y��Һ
Y��Һ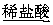 Al(OH)3����
Al(OH)3����
NaOH��Һ��ϡ�����Ũ�Ⱦ���3 mol��L-1,��ͼ����X��Һ��Y��Һ�зֱ����NaOH��Һ��ϡ����ʱ�������������ʵ�����������������������Һ���֮��Ĺ�ϵ,����˵����ȷ����(����)
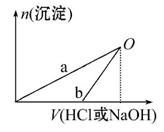
A.X��Һ����ΪAlCl3,Y��Һ����ΪNaAlO2
B.b���߱�ʾ������X��Һ�м���NaOH��Һ
C.��O��ʱ��������������ҺŨ�����
D.a��b���߱�ʾ�ķ�Ӧ����������ԭ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����A�Ļ�ѧʽΪNH5����������ԭ�ӵ�����㶼������Ӧϡ������ԭ�ӵ�������Ӳ�ṹ���������й�˵���У�����ȷ����(����)
A��NH5�м������Ӽ����й��ۼ�
B��NH5���۷е����NH3
C��1 mol NH5�к���5 mol N—H��
D��NH5����Ͷ������ˮ�У��ɲ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з�Ӧ�������ӷ�Ӧ����������ԭ��Ӧ���ǣ���
A�� BaCl2��Һ����ϡ������ B�� ���������ʯ����
C�� ��������CuSO4��Һ�� D�� �����������ͨ��CO
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com