
 ŹµŃéŹŅÓĆ50mL0.50mol/LŃĪĖį”¢50mL0.50mol/LNaOHČÜŅŗŗĶČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠ²ā¶ØÖŠŗĶČȵďµŃ飬µĆµ½±ķÖŠµÄŹż¾Ż£ŗ
ŹµŃéŹŅÓĆ50mL0.50mol/LŃĪĖį”¢50mL0.50mol/LNaOHČÜŅŗŗĶČēĶ¼ĖłŹ¾×°ÖĆ½ųŠŠ²ā¶ØÖŠŗĶČȵďµŃ飬µĆµ½±ķÖŠµÄŹż¾Ż£ŗ| ŹµŃé“ĪŹż |
ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Čt2/”ę | |
| ŃĪĖį | NaOHČÜŅŗ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
| 3.45”ę+3.4”ę+3.35”ę |
| 3 |
| 1.421KJ |
| 0.025 |


 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
 ŹµŃéŹŅÓĆ50mL 0.50mol?L-1ŃĪĖį”¢50mL 0.55mol?L-1 NaOHČÜŅŗŗĶČēĶ¼ĖłŹ¾×°ÖĆ£¬½ųŠŠ²ā¶ØÖŠŗĶČȵďµŃ飬µĆµ½±ķÖŠµÄŹż¾Ż£ŗ
ŹµŃéŹŅÓĆ50mL 0.50mol?L-1ŃĪĖį”¢50mL 0.55mol?L-1 NaOHČÜŅŗŗĶČēĶ¼ĖłŹ¾×°ÖĆ£¬½ųŠŠ²ā¶ØÖŠŗĶČȵďµŃ飬µĆµ½±ķÖŠµÄŹż¾Ż£ŗ| ŹµŃé“ĪŹż | ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Čt2/”ę | |
| ŃĪĖį | NaOHČÜŅŗ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø12·Ö£©ŹµŃéŹŅÓĆ50mL 0.50mol/LµÄŃĪĖįÓė50mL 0.55mol/LµÄÉÕ¼īČÜŅŗ½ųŠŠ·“Ó¦£¬Ķعż²ā¶Ø·“Ó¦¹ż³ĢÖŠµÄ·ÅČČĄ“¼ĘĖćÖŠŗĶČČ”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
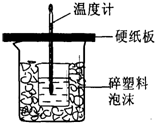
£Ø1£©ŌŚ±¾ŹµŃéÖŠ³żĮĖÓƵ½“óÉÕ±”¢Š”ÉÕ±”¢ĪĀ¶Č¼Ę”¢ĮæĶ²µČŅĒĘ÷Ķā£¬»¹ŠėµÄŅ»ÖÖ²£Į§ŅĒĘ÷ĆūĪŖ ”£
£Ø2£©Į½Ö»ÉÕ±¼äŅŖĢīĀśĖéÖ½Ģõ£¬ĘäÄæµÄŹĒ £»
£Ø3£©“óÉÕ±ÉĻ±ŲŠėøĒÉĻÓ²Ö½°å£¬·ńŌņ£¬ĒóµĆµÄÖŠŗĶČČŹżÖµ½« £ØĢī”°Ę«“ó”±”¢”°Ę«Š””±”¢”°ĪŽÓ°Ļģ”±£©”£
£Ø4£©ŹµŃ鏱ĖłÓĆŃĪĖį¼°NaOHČÜŅŗµÄĢå»ż¾łĪŖ50mL£¬ø÷ČÜŅŗĆܶČĪŖ1g/cm3£¬Éś³ÉČÜŅŗµÄ±ČČČČŻC=4.18J/£Øg”¤”ę£©£¬ŹµŃéĘšŹ¼ĪĀ¶ČĪŖt1”ę£¬ÖÕÖ¹ĪĀ¶ČĪŖt2”ę”£ŹŌĶʶĻÖŠŗĶČČµÄ¼ĘĖćŹ½£ŗ”÷H= ”£
£Ø5£©ŹµŃéÖŠøÄÓĆ52mL0.50mol/LµÄŃĪĖįÓė50mL0.55mol/LµÄÉÕ¼īČÜŅŗ·“Ó¦£¬ÓėÉĻŹöŹµŃéĻą±Č£¬Ėł·Å³öµÄČČĮæ £ØĢī”°ĻąµČ”±”°²»ĻąµČ”±£©£¬ĖłĒóÖŠŗĶČČ £ØĢī”°ĻąµČ”±”°²»ĻąµČ”±£©¼ņŹöĄķÓÉ
£Ø6£©ÓĆĻąĶ¬ÅضČŗĶĢå»żµÄ°±Ė®“śĢęÉÕ¼īČÜŅŗ½ųŠŠÉĻŹöŹµŃ飬²āµĆµÄÖŠŗĶČȵďżÖµ»į £»ČōøÄÓĆ50mL 0.50mol/LÉÕ¼īČÜŅŗ½ųŠŠÉĻŹöŹµŃ飬²āµĆµÄÖŠŗĶČȵďżÖµ»į ”££ØĢī”°Ę«“ó”±”¢”°Ę«Š””±”¢”°ĪŽÓ°Ļģ”±£©
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĪŹ“šĢā
| ŹµŃé“ĪŹż |
ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Čt2/”ę | |
| ŃĪĖį | NaOHČÜŅŗ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |
²éæ““š°øŗĶ½āĪö>>
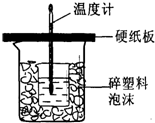
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012-2013ѧğÕć½Ź”ŗ¼ÖŻŹŠĘߊ£ĮŖæ¼ø߶ž£ØĻĀ£©ĘŚÖŠ»ÆѧŹŌ¾ķ£Ø½āĪö°ę£© ĢāŠĶ£ŗĢīæÕĢā
| ŹµŃé“ĪŹż | ĘšŹ¼ĪĀ¶Čt1/”ę | ÖÕÖ¹ĪĀ¶Čt2/”ę | |
| ŃĪĖį | NaOHČÜŅŗ | ||
| 1 | 20.2 | 20.3 | 23.7 |
| 2 | 20.3 | 20.5 | 23.8 |
| 3 | 21.5 | 21.6 | 24.9 |

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com