
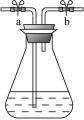
��֪�Ǽ����������ͳ���ɫ,��ԭ�ͳ���ɫ,��ת����ϵʽΪ:������(��ɫ)+ne-![]() ��ԭ��(��ɫ),����ġ���ƿ�ӡ�ʵ�������������ԭ��,��װ����ͼ1��
��ԭ��(��ɫ),����ġ���ƿ�ӡ�ʵ�������������ԭ��,��װ����ͼ1��
ͼ1 ͼ2
ijУ��ѧ��ȤС����ͼ1װ�ý�������ʵ��:
����250 mL��ƿ��,���μ���2 g NaOH��100 mL H2O��2 g������,�����ܽ��,�ټ���3��5��0.2%���Ǽ�����Һ,���Һ����ɫ;
��������Ƥ��,�رջ���a��b,����,��Һ��Ϊ��ɫ;
�۴�������,��Һ�ֱ�Ϊ��ɫ;
�ܹرջ���������,��Һ�ֱ�Ϊ��ɫ;
�����ϲ���ۢܿ��ظ���Ρ�
��ش���������:
(1)������ͼ1����ƿ��,�����ܻ���a��b,��____________���ܿ�(����ҡ�)ͨ������������,�ٹرջ���a��b����,��Һ_________(��ܡ����ܡ�)����ɫ��Ϊ��ɫ��
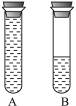
(2)��ͼ2��ʾ:ijѧ���������õ���ɫ��Һ��װ��A��B��֧�Թ���,A�Թܳ�����Һ,B����������Һ,������Ƥ������Ƭ��,����Һ������ɫ������ͬʱ��A��B�Թ�,��Һ����ɫ����_________�Թܡ�
(3)����ת�������������ǵ�������_________,�Ǽ�����������_________��
(4)����ʵ����������Ҳ�����ʳ�֭(���к��ḻά����C)����,������Ϊ____________________________________��
(5)��ʵ���Тۢܲ���_________(��ܡ����ܡ�)�����ظ�����,������______________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(A)�����ʽṹ�����ʡ��γ�ģ��
�±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�

��ش��������⣺
(1)��������ds����Ԫ����___________ (����)��
(2)����Ԫ�آٵ�2��ԭ����Ԫ�آ۵�2��ԭ���γɵķ�����Ԫ�آ۵��ӻ�������________���ۺ͢��γɵij���������Ļ�ѧ��������___________��
(3)Ԫ�آ����Χ�����Ų�ʽΪ___________����Ԫ��ԭ����δ�ɶԵ�����Ϊ___________��
(4)�����ڱ���λ�ڶԽ��ߵ�Ԫ�ص�����Ҳ��һ���������ԡ���д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ��_________________________________________________��
(5)��1 183 K���£�Ԫ�آ��γ���ͼ����ʾ�����ṹ��Ԫ�ľ��壻1 183 K���ϣ�ת��Ϊͼ����ʾ�����ṹ��Ԫ�ľ��塣

��1 183 K���µľ����У���Ԫ�آ��ԭ�ӵȾ����������ԭ����Ϊ___________����1 183 K���ϵľ����У���Ԫ�آ�ԭ�ӵȾ����������ԭ����Ϊ___________��
(B)��ʵ�黯ѧ���γ�ģ��
��֪�Ǽ����������ͳ���ɫ����ԭ�ͳ���ɫ����ת����ϵʽΪ��

������(��ɫ)+ne-![]() ��ԭ��(��ɫ)��
��ԭ��(��ɫ)��
����ġ���ƿ�ӡ�ʵ�������������ԭ������װ����ͼ�ס�
ijУ��ѧ��ȤС����ͼ��װ�ý�������ʵ�飺
����250 mL��ƿ�У����μ���
��������Ƥ�����رջ���a��b�����ã���Һ��Ϊ��ɫ��
�۴���������Һ�ֱ�Ϊ��ɫ��
�ܹرջ��������ã���Һ�ֱ�Ϊ��ɫ��
�����ϲ���ۢܿ��ظ���Ρ�
��ش��������⣺
(1)������ͼ������ƿ���������ܻ���a��b����___________(����ҡ�)���ܿ�ͨ�������������ٹرջ���a��b������Һ__________(��ܡ����ܡ�)����ɫ��Ϊ��ɫ��
(2)��ͼ����ʾ��ijѧ���������õ���ɫ��Һ��װ��A��B��֧�Թ��У�A�Թܳ�����Һ��B����������Һ��������Ƥ������Ƭ�̣�����Һ������ɫ������ͬʱ��A��B�Թܣ���Һ����ɫ����___________�Թܡ�
(3)����ת�������������ǵ�������_______________���Ǽ�����������______________��
(4)����ʵ����������Ҳ�����ʳ�֭(���к��ḻά����C)���棬������Ϊ______________��
(5)��ʵ���Тۢܲ���___________(��ܡ����ܡ�)�����ظ����У�������____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�ء�

��ش��������⣺
(1)��������ds����Ԫ���� (����)��
(2)����Ԫ�آٵ�2��ԭ����Ԫ�آ۵�2��ԭ���γɵķ�����Ԫ�آ۵��ӻ������� ��
�ۺ͢��γɵij���������Ļ�ѧ�������� ��
(3)Ԫ�آ����Χ�����Ų�ʽΪ ����Ԫ��ԭ����δ�ɶԵ�����Ϊ ��
(4)�����ڱ���λ�ڶԽ��ߵ�Ԫ�ص�����Ҳ��һ���������ԡ���д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽ�� .
(5)��1183 K���£�Ԫ�آ��γ���ͼ1��ʾ�����ṹ��Ԫ�ľ��壻1183 K���ϣ�ת�� Ϊͼ2��ʾ�����ṹ��Ԫ�ľ��塣


��1183 K���µľ����У���Ԫ�آ��ԭ�ӵȾ����������ԭ����Ϊ ��
��1183 K���ϵľ����У���Ԫ�آ�ԭ�ӵȾ����������ԭ����Ϊ ��
(B).
��֪�Ǽ����������ͳ���ɫ����ԭ�ͳ���ɫ����ת����ϵʽΪ��
![]()
����ġ���ƿ�ӡ�ʵ�������������ԭ������װ����ͼ1��


ijУ��ѧ��ȤС����ͼ1װ�ý�������ʵ�飺
����250 mL��ƿ�У����μ���2g NaOH��100mlH2O��2g�����ǣ������ܽ���ټ���3��5��0.2�����Ǽ�����Һ�����Һ����ɫ��
��������Ƥ�����رջ���a��b�����ã���Һ��Ϊ��ɫ��
�۴���������Һ�ֱ�Ϊ��ɫ��
�ܹرջ��������ã���Һ�ֱ�Ϊ��ɫ��
�����ϲ���ۡ��ܿ��ظ���Ρ�
��ش��������⣺
(1)������ͼ1����ƿ���������ܻ���a��b���ӵ��ܿ�(����ҡ�)ͨ�������������ٹرջ���a��b������Һ (��ܡ����ܡ�)����ɫ��Ϊ��ɫ��
(2)��ͼ2��ʾ��ijѧ���������õ���ɫ��Һ��װ��A��B��֧�Թ��У�A�Թܳ�����Һ��B����������Һ��������Ƥ������Ƭ�̣�����Һ������ɫ������ͬʱ��A��B�Թܣ���Һ����ɫ���� �Թܡ�
(3)����ת�������������ǵ������� ���Ǽ�����������
(4)����ʵ����������Ҳ�����ʳ�֭(���к��ḻά����C)���棬������Ϊ ��
(5)��ʵ���Тۡ��ܲ��� (��ܡ����ܡ�)�����ظ����У������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com