
���ʴ��ֳ��Ҷ����ѣ�����ʽΪC4H10O3��HO-CH2-CH2-O-CH2-CH2-OH�������ʴ���һ����Ҫ�Ļ���ԭ�ϣ�������ȡ�ᡢ�������ȣ���;ʮ�ֹ㷺�����ʴ�һ��ĺϳ�·�����£�
���̢� Br2 ������ ��Ӧ��
��1�����̢���ʯ�ͼӹ��г��ò��裬������Ϊ ��������B������C�ķ�Ӧ�������� ���÷�Ӧ���� ����д��Ӧ���ͣ�������B������C�Ĺ�������������Ʋ��û���������E��E�����ڽ������ид��B��������E�Ļ�ѧ����ʽ ��
��2��д�������ϳ�·���е�����A��B��C�Ľṹ��ʽ��
A ��B ��C
��3����Ӧ��Ļ�ѧ����ʽΪ�� ��
Br2��
��1���ѽ⣨1�֣���NaOHˮ��Һ��1�֣���ȡ����ˮ�⣩��Ӧ��1�֣���
CH2BrCH2Br + 2NaOH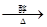 HC��CH��+ 2 NaBr ��1�֣�
HC��CH��+ 2 NaBr ��1�֣�
��2��A��CH2=CH2��B��Br-CH2-CH2-Br��C��HO-CH2-CH2-OH��1��1��3�֣�
��3��2HO-CH2-CH2-OH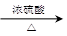 2HO-CH2-CH2-O-CH2-CH2-OH+H2O��1�֣�
2HO-CH2-CH2-O-CH2-CH2-OH+H2O��1�֣�
���������������1��ʯ�͡�����A �� ����B ����ˮ��Ӧ��˵����˫��������Ҫ�ƶ��ʴ�����̼�����٣�ʯ�͡�����A��������̷������ѽⷴӦ���ѽ�������ѻ�������С����ϩ����B��C��BΪ�������C
Ϊ���� ��NaOHˮ��Һ�з�����ˮ�ⷴӦ����ȡ����Ӧ�������и�����Ȳ�������ʡ�
CH2BrCH2Br + 2NaOH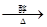 HC��CH��+ 2 NaBr
HC��CH��+ 2 NaBr
��2���ɺϳ�·�����ƶ��ʴ�:2HO-CH2-CH2-O-CH2-CH2-OH��HO-CH2-CH2-OH��Br-CH2-CH2-Br��CH2=CH2
���ԣ�A��CH2=CH2��B��Br-CH2-CH2-Br��C��HO-CH2-CH2-OH
���㣺�������л��ƶ�Ϊ�����������л�����������ʵ�֪ʶ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(10��)�л���A��B��C��D��E��F��һ����ͬ�����ţ�����֮������ͼת����ϵ��
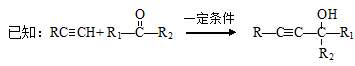
��֪��
�� RCH2OH RCHO
RCHO
��
�ش��������⣺
��1��д��D��F�Ľṹ��ʽ��D ��F
��2��B�������� ��ʵ��C��Eת���ķ�Ӧ������ ��
��3��д��A��������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��4��д��ͬʱ������������������E������ͬ���칹�� �������������칹����
a��������ֻ��һ������ b���ܹ�����ˮ�ⷴӦ c����һ��̼̼˫��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ʽΪC8H8O3�ķ����廯�����ж��ֲ�ͬ�Ľṹ����Щ�����й㷺��;��
��1��C8H8O3��ijһͬ���칹���Ჴ�����Ľṹ��ʽ��ͼ��
�����ж��Ჴ�������ж���ȷ���� ��
a���ܷ���ˮ�ⷴӦ b������FeCl3��Һ������ɫ��Ӧ
c������������ԭ�Ӷ���ͬһƽ���� d������Ũ��ˮ��Ӧ������ɫ������
�� �Ჴ������NaOH��Һ��һ�������·�Ӧ�Ļ�ѧ����ʽ�� ��
��2��C8H8O3����һ��ͬ���칹�����ͼ��
����д���������������ŵ����� �� ��
�ڼ���һ�������¸�Na��Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�ۼ�NaHCO3��Һ��Ӧ�Ļ�ѧ����ʽ�ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
PHBV��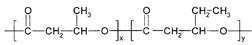 ����һ�ֿɽ���ĸ߷��Ӳ��ϡ������Ʒ������ʳƷ��װ����ױƷ��ҽҩ��������ũҵ����ҵ�������ɻ�Ϊͬϵ���M��N����Ϊ������������ԭ�Ͼ�����·�ߺϳɣ�
����һ�ֿɽ���ĸ߷��Ӳ��ϡ������Ʒ������ʳƷ��װ����ױƷ��ҽҩ��������ũҵ����ҵ�������ɻ�Ϊͬϵ���M��N����Ϊ������������ԭ�Ͼ�����·�ߺϳɣ�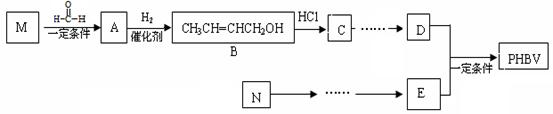
���������գ�
��1��д��N�Ľṹ��ʽ ��C�й����ŵ����� ��
��2��д����Ӧ���ͣ�M��A ��B��ͬ���칹�����ܱ�����������ͭ����Һ�������� �֡�
��3����E��D�ϳ�PHBV�Ļ�ѧ����ʽ�� ��
��4�����Ҵ���ȡ����ķ�������̿ɱ������£�
C2H5OH CH3CHO
CH3CHO CH3COOH
CH3COOH
������������������ʾ��C�ϳ�D�ķ�������̣�
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�л���D��һ�ֺϳɿ���Ѫѹҩ����Ҫͨ���м��壬��ϳ�·�����£�����֪A��һ�ַ����ᣩ
(1) A��C�Ľṹ��ʽ�ֱ���_______��_______��D�к��еĺ���������������_______.
(2) C��D�ķ�Ӧ������_______
(3)���������£�C������NaOHˮ��Һ�з�Ӧ�Ļ�ѧ����ʽ��_______.
(4) E��һ����Է������ñ�AС14�ķ����ᡣд����������������E������ͬ���칹��Ľṹ��ʽ��_______________
���ܷ���������Ӧ ��һ�������¿ɷ���ˮ�ⷴӦ �۷��ӵĺ˴Ź����������������
(5) F��B�ڼ���Һ��ˮ������ữ�IJ��F��һ�������¿ɾۺϳ��{���ӻ����д���÷�Ӧ�Ļ�ѧ����ʽ_____________________.
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
6-�ʻ�������һ����Ҫ�Ļ����м��壬����ϳ���������ͼ��
��֪�� 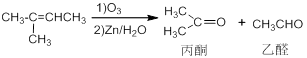
��1����Ӧ�ٵ������� ����Ӧ������ ��
��2������˵������ȷ���� ��
a��1molC��������Na��Ӧ����1molH2 b��C�ܱ���������ͪ
c��Ni����1molG���ֻ����1molH2�ӳ� d��F�ܷ�����ȥ��Ӧ�������ֲ�ͬϩ��
��3��E������Cu(OH)2��Ӧ�Ļ�ѧ����ʽΪ ��
��4��G��ͬ���칹���ж��֡���д���ṹ�к��� �������������ͬ���칹
�������������ͬ���칹
�壺 �� �� ��
��5����֪��Diels-Alder��Ӧ��Ϊ�� ����
����
����D��ૣ�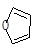 ��Ҳ���Է�����Diels-Alder��Ӧ�����û�ѧ��Ӧ����ʽΪ��
��Ҳ���Է�����Diels-Alder��Ӧ�����û�ѧ��Ӧ����ʽΪ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ƽ�����ھ��������ƣ����ĺϳ�·�����£�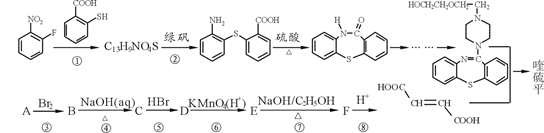
��1��д��C13H9NO4S�����к��������ŵ����� ��
��2��A������������Է���������54��д��A�Ľṹ��ʽ ��
��3����Ӧ�١���������ȡ����Ӧ���� ��ѡ����ţ���
д����Ӧ�ߵĻ�ѧ����ʽ ��
��4����������Ʒ�Ӧ�ݺ͢ߵ�Ŀ���� ��
��5������C��ͬ���칹���ж��֣����мȺ����ǻ����ֺ���ȩ����ͬ���칹���� �֡�
��6����֪�������ϵ��Ȼ�Ϊ��λ��λ������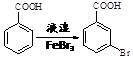 ��д����
��д����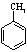 Ϊԭ���Ʊ�
Ϊԭ���Ʊ� �ĺϳ�·������ͼ�����Լ���ѡ�������ñ����е������Ϣ�����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ���ѡ�������ñ����е������Ϣ�����ϳ�·������ͼʾ�����£�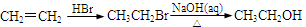 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ʴ���
��5�֣�ij��C��H��O����Ԫ�ص��л���4.6 g��ȫȼ�գ��������ɵ�����ȫ��ͨ��Ũ���ᣬ��Ũ������������5.4 g���������ɵ�����ȫ��ͨ�������Ĺ������ƣ��������Ƶ���������6.2 g���ٶ�����ȫ�����գ���
��1����ͨ�������ƶϸ��л���ķ���ʽ��
��2�����л����ж���ͬ���칹�壬����һ���ܺ�Na��Ӧ����H2������д�����л���Ľṹ��ʽ��________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪ij��ȼ�Ϻ���̼���⡢������Ԫ�ء�Ϊ�˲ⶨ����ȼ����̼��������Ԫ�ص������ȣ��ɽ���̬ȼ�Ϸ���������������ȼ�գ���ʹ����������ȫ��ͨ����ͼ��ʾ��װ�ã��õ����±����е�ʵ����(���������������ȫ������)��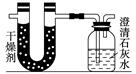
| | ʵ��ǰ | ʵ��� |
| (�������U�ι�)������ | 101.1 g | 102.9 g |
| (ʯ��ˮ�����ƿ)������ | 312.0 g | 314.2 g |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com