
��10�֣�ԭ������֮��Ϊ16�����ֶ�����Ԫ�أ���y������Ӧ�ĵ��� X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ������µ���X��Y��Z֮����Է�������ͼ��ʾ�ı仯����֪B��������У�ԭ�Ӹ�����C��������һ����
��ش��������⣺
��1������Y�ĽṹʽΪ ��
��2��C��X��һ�����������ɻ�����A�Ļ�ѧ����ʽ�� ��
��3�������£�C��ˮ��Һ�ܹ�ʹ��ɫʯ����ֽ�������������ӷ���ʽ��ʾ������ԭ��:
��
��4��д��A��C��Ӧ����Y��B�Ļ�ѧ����ʽ ��
��5���õ���ʽ��ʾB���γɹ��� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
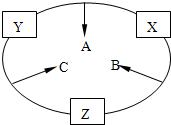 ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯��
ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯��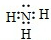
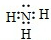
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
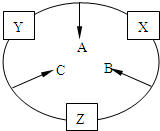 ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯����֪B���������Zԭ��
ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�������ͼ��ʾ�ı仯����֪B���������Zԭ��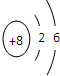
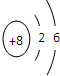
| ||
| �� |
| ||
| �� |
 NH4++OH-
NH4++OH- NH4++OH-
NH4++OH-
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011�갲�պϷ�һ�е���У��һ��ѧ����ĩ������ѧ�Ծ����������� ���ͣ������
(10��)ԭ������֮��Ϊ16�����ֶ�����Ԫ�صĵ���X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ�������X��Y��Z֮����Է�����ͼ��ʾ�ı仯��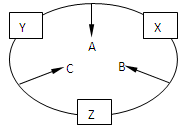
��֪B���������Zԭ�Ӹ�����C��������һ����
��ش��������⣺
(1) Ԫ��Xλ�� ���� ��
(2) Ԫ��Y��ԭ�ӽṹʾ��ͼ
(3) �õ���ʽ��ʾB���γɹ��̣�
(4) B��C���ȶ��Դ�С˳��Ϊ ���û�ѧʽ��ʾ��
(5) C��X��һ�����������ɻ�����A�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������и�һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
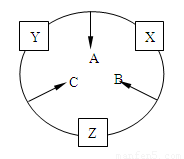
��10�֣�ԭ������֮��Ϊ16�����ֶ�����Ԫ�أ���y������Ӧ�ĵ��� X��Y��Z�����³�ѹ�¾�Ϊ��ɫ���壬���ʵ������µ���X��Y��Z֮����Է�������ͼ��ʾ�ı仯����֪B��������У�ԭ�Ӹ�����C��������һ����
��ش��������⣺
��1������Y�ĽṹʽΪ ��
��2��C��X��һ�����������ɻ�����A�Ļ�ѧ����ʽ�� ��
��3�������£�C��ˮ��Һ�ܹ�ʹ��ɫʯ����ֽ�������������ӷ���ʽ��ʾ������ԭ��:
��
��4��д��A��C��Ӧ����Y��B�Ļ�ѧ����ʽ ��
��5���õ���ʽ��ʾB���γɹ��� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com