
(12��)����ʽΪC3H6O3�������ж���ͬ���칹�壬��д����������Ҫ��ĸ���ͬ���칹��Ľṹ��ʽ(������ͬһ̼ԭ�����������ǻ�)�����ش�������⣺
��1��������û�м�����1 mol������������Na��Ӧ����1 mol H2��������NaHCO3��Һ��Ӧ����Ľṹ��ʽΪ����������������������������������ˮ(��ѡ����ס�)�� ԭ�������������������������������ʵ��۵����Ը�����Է���������ӽ������������۵㣬ԭ������������������������������
��2���ҷ�����̼�����ֱ������ֻ�ѧ��������Ļ�ѧ������ͬ�����������Na����Ӧ�����ҽṹ��ʽΪ�������������������������������������������������(���������ȩ���������ᡱ������)������Һ���ܶ�Ӧ��ˮ����������
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪����A����ʽΪC3H4O2�������ԡ�FΪ���߸�ԭ����ɵĻ�״�ṹ������ʽΪC6H8O4����������¿�ͼ�ش����⣺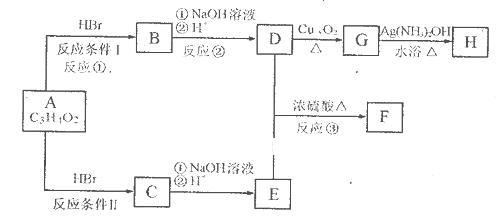
��1��A�Ľṹ��ʽΪ________________________________��
��2����Ӧ�ٵķ�Ӧ����Ϊ__________________________��
��3��������B�к��������ŵ�������______________________________��
��4��D��E����F�Ļ�ѧ����ʽ__________________________________��
D��E����1:1��ӦҲ�����ɸ߾����д�����ɸø߾���Ļ�ѧ��Ӧ����ʽ=______
________________________________________________________________________��
��5��G����H�Ļ�ѧ����ʽ________________________________________________��
��6��д��C��ͬ���칹���������������ʵĽṹ��ʽ_______________________��
________________________��____________________________________������д3����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ҩ�ﰢĪ������ɱ�������ϸ����ֳ�����ĺϳ�·�����£�
��֪��
A��ʹ���Ȼ�����Һ��ɫ��
���������գ�
��1��д��A�Ľṹ��ʽ��_______________��CH3I������_____________________��
��2��д����Ӧ���ͣ���Ӧ��____________����Ӧ��______________��
��3��G�й����ŵ�����_______________��
��4����Ӧ�ܵĻ�ѧ����ʽ______________________________________��
H��һ��ͬ���칹���һ�ȴ���ĽṹΪ ����������NaOHˮ��Һ�м��ȷ�Ӧʱ�Ļ�ѧ����ʽΪ������������������������������������������������
����������NaOHˮ��Һ�м��ȷ�Ӧʱ�Ļ�ѧ����ʽΪ������������������������������������������������
��5����д����ͬʱ������������H���е�ͬ���칹����������������������������������
a�������Ȼ�����Һ������ɫ��Ӧ������b������̼�����Ʒ�Ӧ������ɫ����
c��ȡ0.1mol�л�����������Na��Ӧ�ܲ���3.36L������£�����
d�������ϵ�һ�ȴ���ֻ�����֣�����������ԭ��������3
e�������к��м�
��6��������CH3CH��CH2Ϊԭ���Ʊ�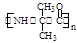 �ĺϳ�·������ͼ�����Լ����ã���
�ĺϳ�·������ͼ�����Լ����ã���
�ϳ�·������ͼ�����£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ѧ����ѡ��5���л���ѧ������ ��15�֣�
��ͼ�� A��B��C��D��E��Ϊ�л��������֪��C�ܸ�NaHCO3������Ӧ���ú�D����Է���������ȣ���EΪ��֧���Ļ����
������ͼ�ش����⣺
��1����֪E����Է�������Ϊ102������̼��������Ԫ�ص����������ֱ�Ϊ58.8%��9.8%������Ϊ����C�����еĹ����������� ______________��������B���ܷ����ķ�Ӧ�ǣ�����ĸ��ţ���______________
a���ӳɷ�Ӧ b��ȡ����Ӧ c����ȥ��Ӧ
d��������Ӧ e��ˮ�ⷴӦ f���û���Ӧ
��2����Ӧ�ڵĻ�ѧ����ʽ��__________________��
��3����Ӧ��ʵ���м��ȵ�Ŀ���ǣ� .��
��4��A�Ľṹ��ʽ�� __________________��
��5��д��ͬʱ������������������B��ͬ���칹������ͬ���칹��Ľṹ��ʽ��
���м��ȡ�������ṹ
�����ڷ������γɵ���
���� FeCl3��Һ������ɫ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��11�֣�������пհס�
��1��������ֵ��������ʾ���͵�������������������ı���̶���С����������ֵ�궨Ϊ100��ͼ
������������ģ�ͣ����������ϵͳ����Ϊ ��
��2��д�����з�Ӧ�Ļ�ѧ����ʽ��
�� 2,5��������2,4������ϩ�����������ӳɣ� ��
�� 2������2����ϩ�Ӿ۷�Ӧ ��
�� �ױ���һ�������������������ױ��� ��
��3��ϩ�����廯�⡢ˮ�ӳ�ʱ������������֮�֣����磺
CH2==CHCH3+HBr��CH3CHBrCH3 (��Ҫ����) + CH3CH2CH2Br(��Ҫ����)
A��һ�ֲ��Գ�ϩ������HBr�ӳ�ʱ�����ɵ���Ҫ����ΪB����B�н�����4��̼ԭ�ӡ�1����ԭ�ӡ�1����ԭ�ӡ���B�Ľṹ��ʽΪ ��A�Ľṹ��ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�����ѧ����ѡ��5���л���ѧ������
��֪����һ��̼ԭ�������������ǻ�ʱ����������ת������R��R�������������ԭ�ӣ���
��ͬһ��̼ԭ������������˫���Ľṹ���ȶ���������ͼ�ش��й����⣺
��1��E�к��еĹ����ŵ������� ��C������������ͭ��Ӧ�Ļ�ѧ����ʽΪ ��
��2��A�Ľṹ��ʽΪ ��A���ܷ����ķ�Ӧ�� ������ĸ����
a��ȡ����Ӧ b����ȥ��Ӧ c��������Ӧ d����ԭ��Ӧ
��3����֪B����Է�������Ϊ188������ȼ�յIJ�����n��CO2����n��H2O����2��1����B�ķ���ʽΪ ��
��4��F���������ص㣺������FeCl3��Һ������ɫ��Ӧ���ں˴Ź�����������ʾ���������շ壻�۱����ϵ�һ�ȴ���ֻ�����֣��ܳ������⣬������������״�ṹ��д���������������������ȶ��ṹ��F���ܵĽṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����ç�п��ס���ʹ���ã�������Ϊ�ϳɿ������Ϳ���ҩ����м��壮ç�������ʵ������ת����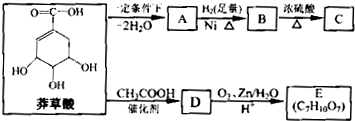
��֪����
��ش��������⣻
��1��A�Ľṹ��ʽ�� ��
��2��B��C�Ļ�ѧ����ʽ�� ����Ӧ������ ��
��3�����л�������У���̼ԭ�������ĸ���ͬ��ԭ�ӻ�ԭ���ţ���̼ԭ���Ϊ����̼ԭ�ӣ�E����������̼ԭ���� ����
��4��ç���ᾭ���м����D�ϳ�E��Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�߷��ӻ�����G����Ϊ﮵����Li+Ǩ�ƵĽ��ʣ��ϳ�G���������£�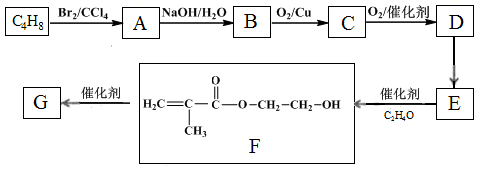
��֪����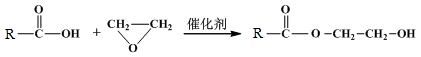
��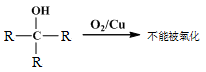
��1��B��������������� ��
��2��A��B�ķ�Ӧ������ ��
��3��C�Ľṹ��ʽ�� ��
��4��D��E��Ӧ����ʽ�� ��
��5��G�Ľṹ��ʽ�� ��
��6��D��һ��ͬ���칹�壬�ܷ���������������Ӧ������Ԫ��״�������ͬ���칹��Ľṹ��ʽ�� ��
��7����֪��
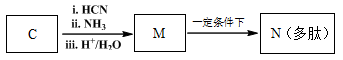
M��N�Ļ�ѧ����ʽ�� ��
��8������˵����ȷ���� (����ĸ)��
a��E��˳���칹��
b��C�ܷ����ӳɡ���ȥ��Ӧ
c��M�������ᷴӦ��������Ӧ
d�������� C��Ӧ���γɸ߷��ӻ�����
e������-OH��-COOH��D��ͬ���칹����2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��15�֣�ij�о�С���Լױ�Ϊ��Ҫԭ�ϣ���������·�ߺϳ�ҽҩ�м���F��Y��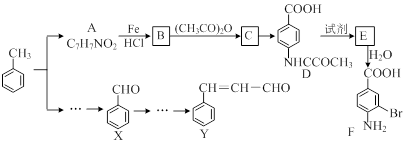
��֪����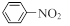
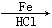

��2CH3CHO 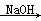 CH3CH(OH)CH2CHO
CH3CH(OH)CH2CHO  CH3CH��CHCHO
CH3CH��CHCHO
��ش��������⣺
�������й�F��˵����ȷ������ ����
| A������ʽ��C7H7NO2Br |
| B��������� |
| C���ܷ���ȡ����Ӧ�����۷�Ӧ |
| D��1 mol�� F�����Ժ�2 mol NaOH��Ӧ |
 CH3COOH
CH3COOH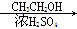 CH3COOCH2CH3
CH3COOCH2CH3�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com