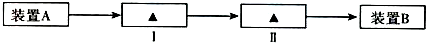
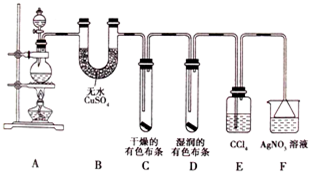
ij��ѧ������ȤС��Ϊ̽��ͭ��Ũ����ķ�Ӧ���������ͼ1��ʾװ�ý����й�ʵ�飮
��֪����SO
2�����ڱ���������������Һ����SO
2�������Ը��������Һ����������ԭ��Ӧ��
��ش�
��1��װ��A�з����Ļ�ѧ��Ӧ����ʽΪ
Cu+2 H
2SO
4��Ũ��
CuSO
4+SO
2��+2 H
2O
Cu+2 H
2SO
4��Ũ��
CuSO
4+SO
2��+2 H
2O
���˷�Ӧ����Ũ������У�����ĸ��
ac
ac
��
a������ b����ˮ�� c��ǿ������ d����ˮ��
��2��װ��D���Թܿڷ��õ����н���
NaOH
NaOH
��Һ����������
����SO2���壬��ֹ��Ⱦ����
����SO2���壬��ֹ��Ⱦ����
��
��3��װ��B�������������������壮��D�������Ե�����ر�����K����ȥ�ƾ��ƣ����������ȵ����ã�A�����������������ʱB�е�������
����ƿ��Һ���½�������©����Һ������
����ƿ��Һ���½�������©����Һ������
��B��Ӧ���õ�Һ���ǣ�����ĸ��
b
b
��
a��ˮ b������NaHSO
3��Һ c������KMnO
4��Һ d��NaOH��Һ
��4��ʵ���У�ȡһ��������ͭƬ��һ�����18.4mol?L
-1��Ũ�������Բ����ƿ�й��ȣ�ֱ����Ӧ��ϣ�������ƿ�л���ͭƬʣ�࣬��С��ѧ��������ѧ�Ļ�ѧ֪ʶ��Ϊ����һ����������ʣ�࣮
����һ���������ᵫδ��ʹͭƬ��ȫ�ܽ⣬����Ϊԭ����
Ũ������Ũ��ϡ��ϡ�������ͭ��Ӧ
Ũ������Ũ��ϡ��ϡ�������ͭ��Ӧ
��
������ҩƷ��������֤����Ӧ���������ƿ��ȷ��������ǣ�����ĸ��
b��d
b��d
��
a������ b������ c��BaCl
2��Һ d��NaHCO
3��Һ
��5��ʵ���з����Թ��ڳ��˲�����ɫ�����⣬��ͭ���滹������ɫ����ף����п��ܺ�������ͭ����ͭ������ͭ���Լ����ڱε�������ͭ��
�������ϣ�
i��������ͭ�����Ի����»ᷢ������������ԭ��Ӧ����Cu
2+��ͭ���ʣ��������������գ�����ת��Ϊ����ͭ��
ii����ͭ������ͭ�����¶�������ϡ���ᣬ�������������գ���ͭ������ͭ��ת��Ϊ����ͭ�Ͷ�������
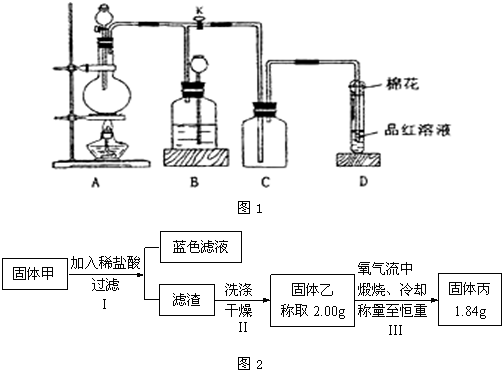
Ϊ���о��ijɷ֣���С��ͬѧ���ռ����㹻���Ĺ����������ͼ2��ʾʵ�飺
�٢��������չ�����һ�������ķ�Ӧ�Ļ�ѧ����ʽΪ
��
�����ж��ڹ���ijɷֵ��ж��У���ȷ���ǣ�����ĸѡ�
a c d
a c d
��
a���������CuO��Cu
2O������һ��
b���������CuS��Cu
2S����ͬʱ����
c�����������û��Cu
2O����һ����Cu
2S
d���������������Cu
2O��Ҳ������Cu
2S��

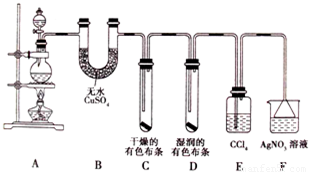


 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
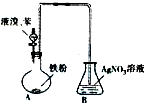 ij��ѧ������ȤС��Ϊ̽�������巢����Ӧ�ķ�Ӧ���Ͳ���ȡ�������屽����������ʵ�飮�����Ҫ��ش�������⣮
ij��ѧ������ȤС��Ϊ̽�������巢����Ӧ�ķ�Ӧ���Ͳ���ȡ�������屽����������ʵ�飮�����Ҫ��ش�������⣮