
�� �� | Al | Al2O3 | Fe | Fe2O3 |
�۵�/�� | 660 | 2 054 | 1 535 | 1 462 |
�е�/�� | 2 467 | 2 980 | 2 750 | �� |
I����1��ijͬѧ�Ʋ⣬���ȷ�Ӧ���õ���������Ӧ�������Ͻ������ǣ��÷�Ӧ�ų�������ʹ���ۻ����������۵�����ͣ���ʱҺ̬���������ۺ��γ������Ͻ�����Ϊ���Ľ����Ƿ��������___________���������������������
��2�����һ����ʵ�鷽����֤���������õĿ�״���������Ƿ��н���������ʵ�������Լ���_____________�����ܷ�����Ӧ�����ӷ���ʽΪ______________________________��
��3��ʵ�����ܽ������������Լ�����õ���___________������ţ���
A.Ũ���� B.ϡ���� C.ϡ���� D.����������Һ
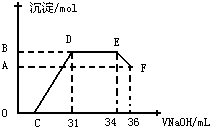
��.ʵ���о����֣����ᷢ��������ԭ��Ӧʱ�������Ũ��Խϡ����Ӧ��ԭ�����е�Ԫ�صĻ��ϼ�Խ�͡�ijͬѧȡһ������������������һ������ϡ�������ַ�Ӧ����Ӧ������������ų����ڷ�Ӧ���������Һ�У���μ���4 mol��L��1������������Һ����������������Һ����� (mL)������ij��������ʵ���(mol)�Ĺ�ϵ����ͼ��ʾ���Իش��������⣺

��4��ͼ��OC��û�г������ɣ��˽η�����Ӧ�����ӷ���ʽΪ______________________��
��5����DE�Σ����������ʵ���û�б仯����˽η�����Ӧ�����ӷ���ʽΪ_____________����������˵����Һ��____________���OH����������__________ǿ�������ӷ��ţ���
��6��B��A�IJ�ֵΪ_________mol��
��7��B���Ӧ�ij��������ʵ���Ϊ____________mol��C���Ӧ������������Һ�����Ϊ___________mL��
��.(1)����
(2)NaOH��Һ 2Al+2OH��+2H2O====![]() +3H2��
+3H2��
(3)B
��.(4)H+ + OH�� ==== H2O
(5)![]() + OH��
+ OH�� ![]() NH3��H2O Al3����Fe3����H��
NH3��H2O Al3����Fe3����H�� ![]()
(6)0.008
(7)0.0327
��������1�����ȷ�Ӧ�������¶����Խ��������ۻ������Ӧ���γ������Ͻ𡣣�2������֪�����Ŀ�״���������NaOH��Һ�У�һ��ʱ���ȡ������ϴ�ӡ�������������ɸ��������Ƿ��б仯�ó��Ƿ�Ϊ�����Ͻ�Ľ��ۡ����ܷ�����Ӧ�����ӷ���ʽΪ2Al+2OH-+2H2O====![]() +3H2������3����״��������FeΪ����Ӧѡ�������ܽ�Fe�Ҳ������ж������ϡH2SO4��
+3H2������3����״��������FeΪ����Ӧѡ�������ܽ�Fe�Ҳ������ж������ϡH2SO4��
EF�Σ�
Al(OH)3 + OH-==== +2H2O��Al
+2H2O��Al
0.008 mol (36-34)��10-3��4 mol 0.008 mol
B��A�IJ�ֵ��Al(OH)3�����ʵ�����ȣ�Ϊ0.008 mol��
DE�Σ�
![]() + OH-
+ OH- ![]() NH3��H2O
NH3��H2O
0.012 mol (34-31)��10-3��4 mol
��������HNO3�����ã�
![]()
![]()
![]() ��Al
��Al![]() Al3+��Fe
Al3+��Fe![]() Fe3+
Fe3+
�ݵ��ӵ�ʧ�غ��У�
0.012 mol��8=0.008 mol��3+n(Fe)��3
�ã�n��Fe(OH)3��=n(Fe)=0.024 mol
B��Fe(OH)3��Al(OH3)����
0.008 mol+0.024 mol=0.032 mol
�����31 mL�������Ƶ���Һ����NaOH:
31��10
CD��Fe3+��Al3+����NaOH:
3��0.008 mol+3��0.024 mol=0.096 mol
OC��H+����NaOH:
0.124 mol-0.096 mol=0.028 mol
C�����NaOH��Һ�����:
![]() =
=



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | Al | A12O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1565 |
| �е�/�� | 2467 | 2980 | 2750 | ---- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | - |
- 2 |
- 2 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij�о���ѧϰС������ȷ�Ӧʵ��չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�
ij�о���ѧϰС������ȷ�Ӧʵ��չ���о������и��л�ѧ�̲��жԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪��Al��Al2O3��Fe��Fe2O3�۵㡢�е��������£�| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | -- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | Al | Al2O3 | Fe | Fe2O3 |
| �۵�/�� | 660 | 2054 | 1535 | 1462 |
| �е�/�� | 2467 | 2980 | 2750 | -- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��ɽ��ʡ����һ�и����ڶ���������⻯ѧ�Ծ� ���ͣ������
��15�֣���ij��ѧ���̵�ʾ��ͼ��ͼ��ʾ����װ�ù��������У��׳ص��ܷ�ӦʽΪ�� ��
��
�Իش��������⣺
��1���׳���Һ�е� ���� ���a����b�����缫��
���� ���a����b�����缫��
�ҳ���Һ�е� ���� ��� A����B�����缫��
���� ��� A����B�����缫��
��2���缫a�Ϸ����ĵ缫��ӦʽΪ �� ��3���ҳ��з�����Ӧ�����ӷ���ʽΪ ��
��3���ҳ��з�����Ӧ�����ӷ���ʽΪ ��
��4�����缫A���õ�0.71g����ʱ���׳�������������
 (��״����)����μ���A���IJ��� ��
(��״����)����μ���A���IJ��� ��
��ij�о���ѧϰС������ȷ�Ӧʵ��չ���о������и��л�ѧ�̲Ķԡ����ȷ�Ӧ��������������������������Ӧ�ų��������ȣ�������ҫ�۵Ĺ�â������ֽ©�����²����մ���������������ɳ�С������ġ���ѧ�ֲᡷ֪�� �۵㡢�е��������£�
�۵㡢�е��������£�
| ���� | Al |  |  |  |
| �۵�/�� | 660 | 2054 | 153 | 14 62 62 |
| �е�/�� | 2467 | 2980 | 2 750 750 | - |
 ��
�� �Լ��������˵��Լ���
�Լ��������˵��Լ��� �鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com